��Ŀ����
��ѧ��ѧ�г�������������ͼ��ʾ��ת����ϵ����Ӧ������ȥ����X��Y��Z��W�ǵ��ʣ������Ϊ�����A��W��Z�����³���̬����A��һ�ִ�����Ⱦ�B��һ�ֳ��õ��ᣮ
��1��д���������ʵĻ�ѧʽ��
Y______��A______��C______��
��2��D�ĵ���ʽΪ______��
��3���ҹ�����B�Ĺ�ҵ�У����������·�ʽ����β��A��
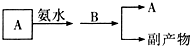
�Դ��ۺϾ���Ч��ĽǶȷ���������������Ŀ���ǣ�����������㼴�ɣ���
a��______��b��______��
��4����Ӧ�ٵĻ�ѧ����ʽ��______��
��Ӧ�ڵĻ�ѧ����ʽ��______��
��Ӧ�ܵ����ӷ���ʽ��______��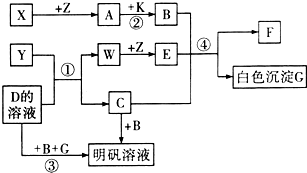
��1��д���������ʵĻ�ѧʽ��
Y______��A______��C______��
��2��D�ĵ���ʽΪ______��
��3���ҹ�����B�Ĺ�ҵ�У����������·�ʽ����β��A��
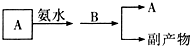
�Դ��ۺϾ���Ч��ĽǶȷ���������������Ŀ���ǣ�����������㼴�ɣ���
a��______��b��______��
��4����Ӧ�ٵĻ�ѧ����ʽ��______��
��Ӧ�ڵĻ�ѧ����ʽ��______��
��Ӧ�ܵ����ӷ���ʽ��______��

A��һ�ִ�����Ⱦ�����ΪSO2��NO2������Ŀ��֪��X��Z��ֻ��ZΪ���壬��XӦΪS��ZΪO2��AΪSO2��B��һ�ֳ��õ��ᣬӦΪH2SO4������SO2��K��Ӧ����H2SO4����KӦΪ����������H2O2��H2SO4��C��Ӧ������������CӦΪKAlO2������Y��DӦΪAl��KOH��Һ�ķ�Ӧ����YΪAl��DΪKOH��WΪH2��EΪH2O��������GΪAl��OH��3��FΪK2SO4����
��1�������Ϸ�����֪��YΪAl��AΪSO2��CΪKAlO2���ʴ�Ϊ��Al��SO2��KAlO2��
��2��DΪKOH��Ϊ���ӻ��������ʽΪ
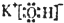
���ʴ�Ϊ��
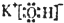
��
��3����������Ͱ�ˮ��Ӧ����������泥�������������ⷴӦ��������泥��ɼ���SO2�Ի�����Ⱦ��ʹԭ�������ʵõ���ߡ�����Ʒ�������ʣ�
�ʴ�Ϊ��a������SO2�Ի�����Ⱦ��b������ʹԭ�������ʵõ���ߣ�c������Ʒ�������ʣ���������������������֣�
��4����Ӧ��Al��KOH��Һ�ķ�Ӧ����Ӧ�Ļ�ѧ����ʽΪ2Al+2KOH+2H2O=2KAlO2+3H2������Ӧ��ΪH2O2��SO2�ķ�Ӧ����Ӧ�Ļ�ѧ����ʽΪH2O2+SO2=H2SO4����Ӧ��KAlO2����ķ�Ӧ������Al��OH��3��������Ӧ�����ӷ���ʽΪAlO2-+H++H2O=Al��OH��3��
�ʴ�Ϊ��2Al+2KOH+2H2O=2KAlO2+3H2����H2O2+SO2=H2SO4��AlO2-+H++H2O=Al��OH��3����
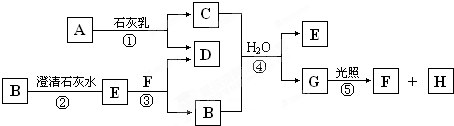
��1�������Ϸ�����֪��YΪAl��AΪSO2��CΪKAlO2���ʴ�Ϊ��Al��SO2��KAlO2��
��2��DΪKOH��Ϊ���ӻ��������ʽΪ
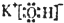
���ʴ�Ϊ��
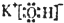
��
��3����������Ͱ�ˮ��Ӧ����������泥�������������ⷴӦ��������泥��ɼ���SO2�Ի�����Ⱦ��ʹԭ�������ʵõ���ߡ�����Ʒ�������ʣ�
�ʴ�Ϊ��a������SO2�Ի�����Ⱦ��b������ʹԭ�������ʵõ���ߣ�c������Ʒ�������ʣ���������������������֣�
��4����Ӧ��Al��KOH��Һ�ķ�Ӧ����Ӧ�Ļ�ѧ����ʽΪ2Al+2KOH+2H2O=2KAlO2+3H2������Ӧ��ΪH2O2��SO2�ķ�Ӧ����Ӧ�Ļ�ѧ����ʽΪH2O2+SO2=H2SO4����Ӧ��KAlO2����ķ�Ӧ������Al��OH��3��������Ӧ�����ӷ���ʽΪAlO2-+H++H2O=Al��OH��3��
�ʴ�Ϊ��2Al+2KOH+2H2O=2KAlO2+3H2����H2O2+SO2=H2SO4��AlO2-+H++H2O=Al��OH��3����

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
 ��ͼ��A��H��Ϊ��ѧ��ѧ�г��������ʣ�����֮��������ת����ϵ������A��C��Ϊ�������ʣ�C��ˮ��Ӧ����D����������壬D��H����ɫ��Ӧ���ʻ�ɫ����ͨ��״����E�����������NaOH��������ɷ������ֽⷴӦ����Ӧ���������ɵ�ˮ��������������ȥ����
��ͼ��A��H��Ϊ��ѧ��ѧ�г��������ʣ�����֮��������ת����ϵ������A��C��Ϊ�������ʣ�C��ˮ��Ӧ����D����������壬D��H����ɫ��Ӧ���ʻ�ɫ����ͨ��״����E�����������NaOH��������ɷ������ֽⷴӦ����Ӧ���������ɵ�ˮ��������������ȥ����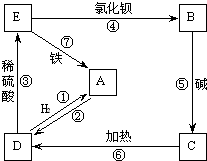 ����A��E����ͬ��Ԫ�أ�������ѧ��ѧ�г��������ʣ����ǿɷ�����ͼ����ʾ�ķ�Ӧ����A��E���������������ȥ����
����A��E����ͬ��Ԫ�أ�������ѧ��ѧ�г��������ʣ����ǿɷ�����ͼ����ʾ�ķ�Ӧ����A��E���������������ȥ����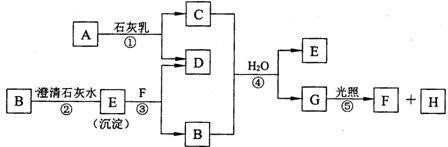
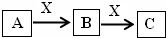 A��B��C��X����ѧ��ѧ�г��������ʣ�����֮���ת����ϵ��ͼ��ʾ�����ֲ�������ȥ������ش��������⣺
A��B��C��X����ѧ��ѧ�г��������ʣ�����֮���ת����ϵ��ͼ��ʾ�����ֲ�������ȥ������ش��������⣺