��Ŀ����
��12�֣�ij��ѧ��ȤС��������ͼ��ʾԭ���װ�ý���ʵ�飬��ش��������⣺

��1��ʵ���У�ͬѧ�Ƿ�����װ�õ����Ƶ�ָ��ƫת����ͬ���������������¹۵㣬������ȷ����____________����˫ѡ�⣬©ѡ��1�֣���ѡ��ѡ��0�֣�
��2��д����ͼ�еĵ缫��Ӧʽ��
��������__________________________________________��
�ڸ�����__________________________________________��
��3����ͼ����Ƭ��NaOH��Һ��Ӧ���ܻ�ѧ����ʽΪ______________________��ijͬѧ�����ͼ��ʵ��ǰ��Ƭ��������10g��ʵ����������4.6g������ʵ������в������������Ϊ L���������ת�Ƶ��ӵ����ʵ���Ϊ mol��

��1��ʵ���У�ͬѧ�Ƿ�����װ�õ����Ƶ�ָ��ƫת����ͬ���������������¹۵㣬������ȷ����____________����˫ѡ�⣬©ѡ��1�֣���ѡ��ѡ��0�֣�
| A�������������þǿ |
| B���������þ����ǿ����װ����þ��Ϊ���� |
| C�������ݽ������˳����ȷ�ж�ԭ��ص������� |
| D��ԭ����е��������ܵ������Һ������ԡ�ǿ�����Ե����ص�Ӱ�� |
��������__________________________________________��
�ڸ�����__________________________________________��
��3����ͼ����Ƭ��NaOH��Һ��Ӧ���ܻ�ѧ����ʽΪ______________________��ijͬѧ�����ͼ��ʵ��ǰ��Ƭ��������10g��ʵ����������4.6g������ʵ������в������������Ϊ L���������ת�Ƶ��ӵ����ʵ���Ϊ mol��
��1��CD��˫ѡ�⣬©ѡ��1�֣���ѡ��ѡ��0�֣�
��2����2H+��2e�D==H2���� ��Mg - 2e�D==Mg2��
��3��2Al + 2NaOH + 2H2O = 2NaAlO2 + 3H2�� 6.72L ��0.6mol
��2����2H+��2e�D==H2���� ��Mg - 2e�D==Mg2��
��3��2Al + 2NaOH + 2H2O = 2NaAlO2 + 3H2�� 6.72L ��0.6mol
��1����һ������£�ԭ����нϻ��õĽ������������ϲ����õĽ��������������ǻ���Ҫ�������õĵ������Һ������þ���������Ʋ���Ӧ�������������Ʒ�Ӧ�����Դ�ʱ��������������������þ��þ��������������ȷ�Ĵ���CD��
��2����װ����þ�Ǹ�����ʧȥ���ӡ�������������Һ�е������ӵõ����ӣ�����������
��3�������������Ʒ�Ӧ����������ƫ�����ƺ�ˮ����ӦʽΪ2Al + 2NaOH + 2H2O = 2NaAlO2 + 3H2�������������֪�����ĵ�����10g��4.6g��5.4g�����ʵ�����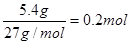 ����������������0.3mol��ת�Ƶ�����0.2mol��3��0.6mol��
����������������0.3mol��ת�Ƶ�����0.2mol��3��0.6mol��
��2����װ����þ�Ǹ�����ʧȥ���ӡ�������������Һ�е������ӵõ����ӣ�����������
��3�������������Ʒ�Ӧ����������ƫ�����ƺ�ˮ����ӦʽΪ2Al + 2NaOH + 2H2O = 2NaAlO2 + 3H2�������������֪�����ĵ�����10g��4.6g��5.4g�����ʵ�����
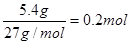 ����������������0.3mol��ת�Ƶ�����0.2mol��3��0.6mol��
����������������0.3mol��ת�Ƶ�����0.2mol��3��0.6mol��
��ϰ��ϵ�д�
 �Ƹ������������ϵ�д�
�Ƹ������������ϵ�д�
�����Ŀ

 ��7H2O
��7H2O CO2+2H2O
CO2+2H2O

