��Ŀ����
5���������������з���Ļ����ڼ�����ѡ�����õ��Լ���������ѡ�����õ���Ҫ����������������������Ӧ���У����������Լ��������á�/����ʾ�����飺��ˮ ������������Һ �����Ȼ�̼ ��ʳ��
���飺A���ᾧ B������ C����ȡ D����Һ
| ��� | ����� | �Լ���� | ������� |
| ��1�� | ����غ��Ȼ��ƵĹ������� | ||
| ��2�� | �Ȼ��غ���Ļ��Һ | ||
| ��3�� | ���ͺ�ˮ�Ļ���� | ||
| ��4�� | �Ҷ������е�198�棩�ͱ��������е�290�棩�Ļ���� |
���� ��1������غ��Ȼ��Ƶ��ܽ�����¶ȵı仯��ͬ��
��2�������������Ȼ�̼��
��3�����ͺ�ˮ�ֲܷ㣻
��4���Ҷ����ͱ������ķе㲻ͬ��
��� �⣺��1������غ��Ȼ��Ƶ��ܽ�����¶ȵı仯��ͬ���ɼ���ˮ�ܽ⣬Ȼ���ýᾧ�ķ������룻
��2�������������Ȼ�̼����KCl���ܣ�������ȡ���룻
��3�����ͺ�ˮ�ֲܷ㣬���÷�Һ���룻
��4���Ҷ����ͱ������ķе㲻ͬ������������룻
�ʴ�Ϊ��
| ��� | ����� | �Լ���� | ������� |
| ��1�� | ����غ��Ȼ��ƵĹ������� | �� | A |
| ��2�� | ��ˮ | �� | C |
| ��3�� | ���ͺ�ˮ�Ļ���� | / | D |
| ��4�� | �Ҷ������е�198�棩�ͱ��������е�290�棩�Ļ���� | / | B |
���� ���⿼�����ʵķ��롢�ᴿ֪ʶ����Ŀ�ѶȲ���ע��������ʵ����ʵ���ͬѡ��ʵ�鷽����ѧϰ��Ҫע�����֪ʶ�Ļ��ۣ�

��ϰ��ϵ�д�
�����Ŀ
15�����г�ȥ���ʵķ����д�����ǣ�������
| ���� | ���� | �����ʵķ��� | |
| A | CaCl2��Һ | HCl | ����CaCO3������ |
| B | NaOH��Һ | Ca��OH��2 | ����Na2CO3��Һ������ |
| C | FeCl2��Һ | CuCl2 | �������ۡ����� |
| D | CH4 | H2O | ͨ��ʢŨ�����ϴ��ƿ |
| A�� | A | B�� | B | C�� | C | D�� | D |
16��������Һ����Ũ�ȹ�ϵһ����ȷ���ǣ�������
| A�� | ��ˮ���ȵ�100�棬pH=6��c��H+����c��OH-�� | |
| B�� | 0.1mol•L-1 ���������Һ�У�c��NH4+����c��SO42-����c��H+�� | |
| C�� | �����£�pH=7�Ĵ���ʹ����ƵĻ����Һ�У�c��CH3COO-����c��Na+�� | |
| D�� | ͬŨ�ȵ�������Һ����CH3COONH4��NH4Cl��NH3•H2O�У�c��NH4+���ɴ�С��˳���Ǣڢۢ� |
13�����й�����ϴ��������������У���ȷ���ǣ�������
| A�� | �Թ�������������ȵĴ�����Һϴ��������ˮ��ϴ | |
| B�� | �Ȱ��Թ���ķ�Һ������ˮ���У�������ˮ��ϴ | |
| C�� | �������������������Թܣ������ռ��ܽ⣬������ˮ��ϴ | |
| D�� | ʢ��ʯ��ˮ������¹������ʵ��ձ������÷���ˮϴ��������ˮ��ϴ |
20������ʵ��������ʵ��ԭ������۾���ȷ���ǣ�������
| ѡ�� | ʵ������ | ʵ��ԭ������� |
| A | ����ɫNO2��ѹ����С�������ɫ���� | 2NO2��g��?N2O4��g��ƽ�������ƶ�c��NO2��Ũ������ |
| B | ��ˮ�˱����ڱܹ������� | Cl2+H2O?HClO+HCl������HClO�ֽ⣬ƽ�������ƶ�����ˮ���� |
| C | ����KMnO4��H2C2O4��Һ��Ӧ��һ��ʱ���Ӧ���������ӿ� | 2MnO4-+5H2C2O4+6H+�T2Mn2++10CO2��+8H2O |
| D | Fe��ϡ����ķ�Ӧ���μӼ�������ͭ��Һ����Ӧ���ʼӿ� | Cu2+�Ƿ�Ӧ�Ĵ����������˷�Ӧ�Ļ�ܣ��ӿ컯ѧ��Ӧ���� |
| A�� | A | B�� | B | C�� | C | D�� | D |
17���������ᷴӦ�����ܲ����������ǣ�������
| A�� | ��ˮ | B�� | Na2S��Һ | C�� | SO2 | D�� | ϡH2SO4 | ||||
| E�� | Fe3+ |
12���������һ�����ᣬ��������ʴ��������֪25��ʱ
��HF��aq��+OH- ��aq��=F- ��aq��+H2O��l����H=-67.7KJ•mol-1
��H+��aq��+OH- ��aq��=H2O��l����H=-57.3KJ•mol-1
��20mL0.1•molL-1������м���VmL0.1mol•L-1NaOH��Һ�������й�˵����ȷ���ǣ�������
��HF��aq��+OH- ��aq��=F- ��aq��+H2O��l����H=-67.7KJ•mol-1
��H+��aq��+OH- ��aq��=H2O��l����H=-57.3KJ•mol-1
��20mL0.1•molL-1������м���VmL0.1mol•L-1NaOH��Һ�������й�˵����ȷ���ǣ�������
| A�� | �����ĵ��뷽��ʽ����ЧӦ�ɱ�ʾΪ��HF��aq��?H+��aq��+F-��aq����H=+10.4 KJ•mol-1 | |
| B�� | ��V=20ʱ����Һ�У�c��F-����c��Na+��=0.1 mol?L-1 | |
| C�� | ��V=20ʱ����Һ�У�c��Na+����c��F-����c��OH-����c��H+�� | |
| D�� | ��V��0ʱ����Һ��һ�����ڣ�c��Na+����c��F-����c��OH-����c��H+�� |
13���뵼�������г���Ҫ���Ʋ��ӣ��Ա�֤���Ƶ����ʣ����Ȼ��ף�PCl3����һ����Ҫ�IJ��Ӽ���ʵ����Ҫ�û��ף������ף�������Cl2������ƿ��ģ�ҵ������ȡPCl3��װ����ͼ��ʾ�������ּг�װ����ȥ��
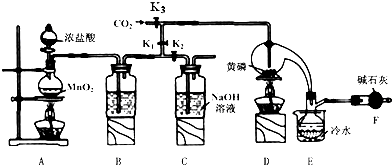
��֪����������Cl2��Ӧ����PCl3�������Cl2��Ӧ����PCl5��PCl3��ˮ��ǿ��ˮ������H3PO3��HCl����O2������POCl3��POCl3����PCl3��PCl3��POCl3���۷е������
��ش��������⣺
��1��B����װ�Լ���ŨH2SO4��E����ˮ������������PCl3��ֹ��ӷ���
��2��F�м�ʯ�ҵ����������ն������������ֹ�����е�ˮ����������ƿ�к�PCl3 ��Ӧ��
��3��ʵ��ʱ�����װ�������Ժ��ȴ�K3ͨ������CO2����Ѹ�ټ�����ף�ͨ����CO2���������ž�װ���еĿ�������ֹ������ȼ��
��4���ֲ�Ʒ�г�����POC13��PCl5�ȣ���������ȳ�ȥPCl5��ͨ��������ʵ��������ƣ������ɵõ��ϴ�����PCl3��
��5��ʵ�����ʱ����������C�е��Լ����ն����������C�з�Ӧ�����ӷ���ʽΪCl2+2OH-=Cl-+ClO-+2H2O��
��6��ͨ�����淽���ɲⶨ��Ʒ��PCl3������������
��Ѹ�ٳ�ȡ1.00g��Ʒ����ˮ��Ӧ�����250mL��Һ��
��ȡ������Һ25.00mL�������м���10.00mL 0.1000mol/L��ˮ����ַ�Ӧ��
�����������Һ�м��뼸�ε�����Һ����0.1000mol/L��Na2S2O3����Һ�ζ���
���ظ��ڡ��۲�����ƽ������Na2S2O3��Һ8.40mL��
��֪��H3PO3+I2=H3PO4+2HI��I2+2Na2S2O3=2NaI+Na2S4O6��
�����������ݣ�����ⶨ������û��������Ӧ���ò�Ʒ��PCl3����������Ϊ79.75%��

��֪����������Cl2��Ӧ����PCl3�������Cl2��Ӧ����PCl5��PCl3��ˮ��ǿ��ˮ������H3PO3��HCl����O2������POCl3��POCl3����PCl3��PCl3��POCl3���۷е������
| ���� | �۵�/�� | �е�/�� |
| PCl3 | -112 | 75.5 |
| POCl3 | 2 | 105.3 |
��1��B����װ�Լ���ŨH2SO4��E����ˮ������������PCl3��ֹ��ӷ���
��2��F�м�ʯ�ҵ����������ն������������ֹ�����е�ˮ����������ƿ�к�PCl3 ��Ӧ��
��3��ʵ��ʱ�����װ�������Ժ��ȴ�K3ͨ������CO2����Ѹ�ټ�����ף�ͨ����CO2���������ž�װ���еĿ�������ֹ������ȼ��
��4���ֲ�Ʒ�г�����POC13��PCl5�ȣ���������ȳ�ȥPCl5��ͨ��������ʵ��������ƣ������ɵõ��ϴ�����PCl3��
��5��ʵ�����ʱ����������C�е��Լ����ն����������C�з�Ӧ�����ӷ���ʽΪCl2+2OH-=Cl-+ClO-+2H2O��
��6��ͨ�����淽���ɲⶨ��Ʒ��PCl3������������
��Ѹ�ٳ�ȡ1.00g��Ʒ����ˮ��Ӧ�����250mL��Һ��
��ȡ������Һ25.00mL�������м���10.00mL 0.1000mol/L��ˮ����ַ�Ӧ��
�����������Һ�м��뼸�ε�����Һ����0.1000mol/L��Na2S2O3����Һ�ζ���
���ظ��ڡ��۲�����ƽ������Na2S2O3��Һ8.40mL��
��֪��H3PO3+I2=H3PO4+2HI��I2+2Na2S2O3=2NaI+Na2S4O6��
�����������ݣ�����ⶨ������û��������Ӧ���ò�Ʒ��PCl3����������Ϊ79.75%��