��Ŀ����
�ƺ�ݳ��ѳ�Ϊһ��������⣬2013��1��1����ִ�е��½���Ծƺ�ݳ����������Ĵ����涨������ʻ��Ա�����оƾ�Ũ�ȣ�BrAC���ķ����ж��֣�
��1�����������ü���Լ���ɫ�仯�����ж�BrAC���������·�Ӧ���BrAC��
3CH3CH2OH+2KMnO4��3CH3CHO+2MnO2+2KOH+2H2O
������Ӧ�з�����ԭ��Ӧ�Ĺ����� ��
��2����������������������������������ĵ�����Һ���BrAC����Һ�������Ҵ�������Ϊ��ȩ���÷�Ӧ�Ļ�ѧ����ʽΪ ��
��3����֪����2CH3OH��l��+3CO2��g��+4H2O��g����H1=-1275.6kJ?mol-1
��2CO��g��=2CO2��g����H2=-566.0kJ?mol-1
��H20��g��=H2O��l����H3=-44.0kJ?mol-1
д���״�����ȫȼ������һ����̼��Һ̬ˮ���Ȼ�ѧ����ʽ�� ��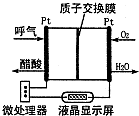
��4����ͼ��һ������ȼ�ϵ�ؾƾ�����ǣ������Զ����������������ƵĹ��ܣ��dz��ʺϽ����ֳ��ƾ���⣮��õ�صĸ�����ӦʽΪ ��������ӦʽΪ ��
��5����0.2mol CO2����ͨ��150mL��2mol?L-1��NaOH��Һ�У���ȫ��Ӧ��������Һ�и�����Ũ���ɴ�С��˳���� ��
��1�����������ü���Լ���ɫ�仯�����ж�BrAC���������·�Ӧ���BrAC��
3CH3CH2OH+2KMnO4��3CH3CHO+2MnO2+2KOH+2H2O
������Ӧ�з�����ԭ��Ӧ�Ĺ�����
��2����������������������������������ĵ�����Һ���BrAC����Һ�������Ҵ�������Ϊ��ȩ���÷�Ӧ�Ļ�ѧ����ʽΪ
��3����֪����2CH3OH��l��+3CO2��g��+4H2O��g����H1=-1275.6kJ?mol-1
��2CO��g��=2CO2��g����H2=-566.0kJ?mol-1
��H20��g��=H2O��l����H3=-44.0kJ?mol-1
д���״�����ȫȼ������һ����̼��Һ̬ˮ���Ȼ�ѧ����ʽ��

��4����ͼ��һ������ȼ�ϵ�ؾƾ�����ǣ������Զ����������������ƵĹ��ܣ��dz��ʺϽ����ֳ��ƾ���⣮��õ�صĸ�����ӦʽΪ
��5����0.2mol CO2����ͨ��150mL��2mol?L-1��NaOH��Һ�У���ȫ��Ӧ��������Һ�и�����Ũ���ɴ�С��˳����
��������1��������������ԭ��Ӧ���ɻ�ԭ�����Ӧ��KMnO4��MnԪ�ػ�������+7�۽���ΪMnO2��+4�ۣ�
��2������������ĵ�����Һ���BrAC���Ҵ�������Ϊ��ȩ���õ��ۼ��������ɣ���Һ��������Ӧ�е����ɣ�
��3������CO��CH3OH���ȷ���ʽ����ϸ�˹�����������״�����ȫȼ������һ����̼��Һ̬ˮ���Ȼ�ѧ����ʽ��
��4������ͼʾ�ó�����ȼ�ϵ�صķ�Ӧ���������ٸ���ԭ���ԭ��д���õ�صķ�Ӧʽ��
��5��������̼���������Ʒ�Ӧ����n��CO2����n��NaOH����1��1ʱ��Ӧ����̼�����ƣ���n��CO2����n��NaOH����1��2ʱ����̼���ƣ���
��n��CO2����n��NaOH����1ʱ����̼���ƺ�̼�����ƣ��ݴ˷������
��2������������ĵ�����Һ���BrAC���Ҵ�������Ϊ��ȩ���õ��ۼ��������ɣ���Һ��������Ӧ�е����ɣ�
��3������CO��CH3OH���ȷ���ʽ����ϸ�˹�����������״�����ȫȼ������һ����̼��Һ̬ˮ���Ȼ�ѧ����ʽ��
��4������ͼʾ�ó�����ȼ�ϵ�صķ�Ӧ���������ٸ���ԭ���ԭ��д���õ�صķ�Ӧʽ��
��5��������̼���������Ʒ�Ӧ����n��CO2����n��NaOH����1��1ʱ��Ӧ����̼�����ƣ���n��CO2����n��NaOH����1��2ʱ����̼���ƣ���
| 1 |
| 2 |
����⣺��1����Ӧ��KMnO4��MnԪ�ػ�������+7�۽���ΪMnO2��+4�ۣ�KMnO4������ԭ��Ӧ����MnO2��
�ʴ�Ϊ��KMnO4��MnO2��
��2������������ĵ�����Һ���BrAC���Ҵ�������Ϊ��ȩ���õ��ۼ��������ɣ���Һ��������Ӧ�е����ɣ���Ӧ����ʽΪ��I2O5+5CH3CH2OH��I2+5CH3CHO+5H2O��
�ʴ�Ϊ��I2O5+5CH3CH2OH��I2+5CH3CHO+5H2O��
��3����2CH3OH��l��+3O2��g��=3CO2��g��+4H2O��g����H1=-1275.6kJ?mol-1
��2CO��g��+O2��g��=2CO2��g����H=-566.0kJ?mol-1
��H20��g��=H2O��l����H3=-44.0kJ?mol-1
�ɸ�˹���ɿ�֪�á���-�ڡ���
+�ۡ�2�÷�ӦCH3OH��l��+O2��g��=CO��g��+2 H2O��l�����÷�Ӧ�ķ�Ӧ�ȡ�H=-726.5kJ?mol-1-��-283.0kJ?mol-1��=-442.8kJ?mol-1��
�ʴ�Ϊ��CH3OH��l��+O2��g��=CO��g��+2 H2O��l����H=-442.8kJ?mol-1��
��4������ͼ��֪����ԭ��ص�ȼ��Ϊ�Ҵ��������������������ˮ�������Ϊ������Һ��ԭ��ظ���ʧȥ���ӷ�����������Ӧ�����Ը�����ӦΪ�Ҵ�ʧȥ�����������ᣬ�缫��ӦΪ��CH3CH2OH+H2O-4e-=CH3COOH+4H+�����������������������õ����ӷ����˻�ԭ��Ӧ��O2+4H++4e-=2H2O��
�ʴ�Ϊ��CH3CH2OH+H2O-4e-=CH3COOH+4H+��O2+4H++4e-=2H2O��
��5���⣺n ��CO2��=0.2mol��150mL��2mol?L-1��NaOH��Һ��n��NaOH��=C��V=0.3mol�����У�2��
=
��1����Ӧ�Ŀ��ܷ���ʽ�У�CO2+2NaOH=Na2CO3+H2O��CO2+NaOH=NaHCO3�����ԣ����ò���ΪNa2CO3��NaHCO3�������ò����к�Na2CO3Ϊxmol��NaHCO3Ϊymol��
��÷����飺
��ã�x=0.1��y=0.1���ں������ʵ�����̼���ơ�̼�����Ƶ���Һ�У�c��Na+�����̼�������ˮ�����̼��������ӵ�ˮ�⣬��c��HCO3-����c��CO32-����ˮ��ʹ��Һ�Լ��ԣ���c��OH-����c��H+�����������Ӵ����������ӣ�����c��HCO3-����c��CO32-����c��OH-����c��H+����
������Ũ�ȴ�СΪc��Na+����c��HCO3-����c��CO32-����c��OH-����c��H+����
�ʴ�Ϊ��c��Na+����c��HCO3-����c��CO32-����c��OH-����c��H+����
�ʴ�Ϊ��KMnO4��MnO2��
��2������������ĵ�����Һ���BrAC���Ҵ�������Ϊ��ȩ���õ��ۼ��������ɣ���Һ��������Ӧ�е����ɣ���Ӧ����ʽΪ��I2O5+5CH3CH2OH��I2+5CH3CHO+5H2O��
�ʴ�Ϊ��I2O5+5CH3CH2OH��I2+5CH3CHO+5H2O��
��3����2CH3OH��l��+3O2��g��=3CO2��g��+4H2O��g����H1=-1275.6kJ?mol-1
��2CO��g��+O2��g��=2CO2��g����H=-566.0kJ?mol-1
��H20��g��=H2O��l����H3=-44.0kJ?mol-1
�ɸ�˹���ɿ�֪�á���-�ڡ���
| 1 |
| 2 |
�ʴ�Ϊ��CH3OH��l��+O2��g��=CO��g��+2 H2O��l����H=-442.8kJ?mol-1��
��4������ͼ��֪����ԭ��ص�ȼ��Ϊ�Ҵ��������������������ˮ�������Ϊ������Һ��ԭ��ظ���ʧȥ���ӷ�����������Ӧ�����Ը�����ӦΪ�Ҵ�ʧȥ�����������ᣬ�缫��ӦΪ��CH3CH2OH+H2O-4e-=CH3COOH+4H+�����������������������õ����ӷ����˻�ԭ��Ӧ��O2+4H++4e-=2H2O��
�ʴ�Ϊ��CH3CH2OH+H2O-4e-=CH3COOH+4H+��O2+4H++4e-=2H2O��
��5���⣺n ��CO2��=0.2mol��150mL��2mol?L-1��NaOH��Һ��n��NaOH��=C��V=0.3mol�����У�2��
| n(NaOH) |
| n(CO2) |
| 3 |
| 2 |
��÷����飺
|
������Ũ�ȴ�СΪc��Na+����c��HCO3-����c��CO32-����c��OH-����c��H+����
�ʴ�Ϊ��c��Na+����c��HCO3-����c��CO32-����c��OH-����c��H+����
���������⿼�������ø�˹���ɽ��з�Ӧ�ȵļ��㡢��ѧ��Դȼ�ϵ�ء�����Ũ�ȴ�С�Ƚϵ�֪ʶ�����ü������з���������̼���������Ʒ�Ӧ����Һ�е������ǽ������ѵ㣬ע��̼�������ˮ��̶ȴ���Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ