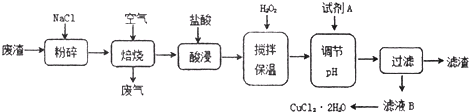
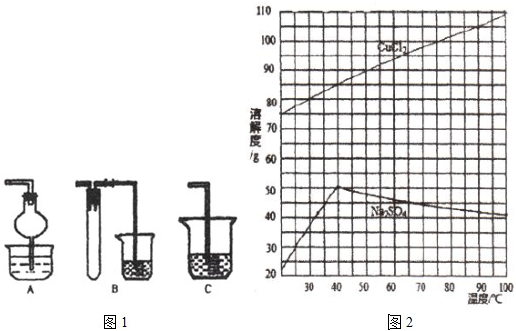
��Ŀ����
��ҵ��Ϊ�˴�������Cr2O72�������Թ�ҵ��ˮ���������еĴ��������� ���̷���FeSO4��7H2O�����ѷ�ˮ�е����۸����ӻ�ԭ�����۸����ӣ��ټ��������ʯ��ˮ��ʹ������ת��ΪCr��OH��3����������Ҫ�Ļ�ѧ����ʽ���£�
H2Cr2O7��6FeSO4��6 H2SO4��Cr2��SO4��3��3Fe 2��SO4��3��7H2O
����������������1��104L��������6�ۣ�78mg/L�ķ�ˮ��Cr���ԭ������Ϊ52������ش��������⣺
�Ŵ����������г�Cr��OH��3�⣬���� . ���û�ѧʽ��ʾ����
�������̷������� ��
��Fe��OH��3 CaSO4����12.51Kg
����:
�ż��������ʯ��ˮʹCr3��ת��ΪCr��OH��3����ȥ��ͬʱFe2��Ҳת����Fe��OH��2��Fe��OH��2���ȶ�������ת��ΪFe��OH��3��������������������SO42����Ca2�������������CaSO4����n��Cr����![]() ��15mol���ɹ�ϵʽ��Cr ��3FeSO4��7H2O�ã�m��FeSO4��7H2O����15mol��3��278g/mol��12510g��12.51Kg
��15mol���ɹ�ϵʽ��Cr ��3FeSO4��7H2O�ã�m��FeSO4��7H2O����15mol��3��278g/mol��12510g��12.51Kg

��ϰ��ϵ�д�
�����Ŀ
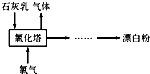 ��������Ҫ�Ļ���ԭ�ϣ�
��������Ҫ�Ļ���ԭ�ϣ�