��Ŀ����
(10��)��.��1�֢�ȥ��̬ԭ���е�һ������ʹ֮��Ϊ��̬+1��������ʱ��������ṩ������������Ԫ�صĵ�һ�����ܡ���ͼ�����ڱ��ж����ڵ�һ���֣����е�һ��������С��Ԫ����_______. �� ����ĸ��
��.��6�֣��¹���������ѧ�������Ƴ���20��̼ԭ����ɵ�
������״����C20������״�ṹ��������������ι��ɣ���ͼ������ش�:
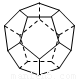
C20���ӹ���_______��������Σ�����_______����ߣ�C20��������_______ (�������).
��. ��3�֣�������й���ļ������Σ���������������ظ���Ԫ��֮Ϊ������NaCl����ṹ��ͼ��ʾ��
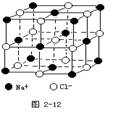
��1��������ÿ��Na+ͬʱ������______��Cl-��ÿ��Cl-ͬʱ������_______��Na+��
��2����������ÿ��Cl-��Χ������ӽ��Ҿ������
��Cl-����________����
��.C(1��)������.12. (2��)30(2��)�����Ӿ���(2��)��
��.6(1��)����6(1��)����12(1��)��
����������.������Խǿ����һ������ԽС������Ԫ�������ɿ�֪C�Ľ�������ǿ�����Ե�һ��������С��
��.���ݽṹͼ��֪��ÿ��̼ԭ���γ�3��C��C����1��C��C����2�������γɣ����Ժ��е������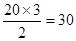 ��ÿ����߱�2�����ڵ�������ι��ã�����ƽ��ÿ���������ӵ��2.5����ߣ����������εĸ�����
��ÿ����߱�2�����ڵ�������ι��ã�����ƽ��ÿ���������ӵ��2.5����ߣ����������εĸ�����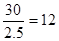 ���û������к��з��ӣ����ڷ��Ӿ��塣
���û������к��з��ӣ����ڷ��Ӿ��塣
��.���ݾ����Ľṹʾ��ͼ��֪ÿ��������ͬʱ������6�������ӣ������¡����ҡ�ǰ���1������������ÿ��������ͬʱ������6�������ӡ�����ʾ��ͼ��֪��������ÿ��Cl-��Χ������ӽ��Ҿ�����ȵ�Cl-λ��������Ĵ�����3��������ÿ����������γ�8�������壬���Ը����� ��
��

(10��)ʵ��С��ⶨij�����е��ܵ�����(�Ե�������������ʾ) ��ʵ��װ������ͼ��ͼ�м��ȼ��г�������ȥ����

��ȡ1.200 g��Ʒ��ͨ����ѧ�����������еĵ�ת������Σ�����Ʒ�⣬�����Լ���������Ԫ�أ���Ȼ���ڼ�����Һ��������25.00 mLŨ��Ϊ0.5000 mol/L-1������Һ������գ�����Ũ��Ϊ0.1000 mol/L-1����������Һ�ζ�δ��Ӧ�����ᡣ�ظ�����ʵ�����Ρ�ʵ���������£�
|
ʵ����� |
����������Һ����� |
|
|
�ζ�ǰ�̶�(mL) |
�ζ���̶�(mL) |
|
|
1 |
0.00 |
5. 01 |
|
2 |
6.00 |
10.99 |
|
3 |
12.00 |
17.60 |
��1��д��Բ����ƿ�ڷ�����Ӧ�����ӷ���ʽ ��
��2����Һ©����Բ����ƿ֮��ĵ���a�������� �� b��������
���������дӣ��n����m���� �ڽ�ˮ��
��3���ڵζ������У����²�����ʹ�����ܵ�����ƫС���ǣ�����ţ����� �������� ��
A����ƿˮϴ��δ��� B���ζ������еζ�����©ˮ����
C���ζ���������������Һ����ƿ�н��� D���ζ�ǰ�ζ����������ݣ��ζ���û����
��4����ʵ���������ȷ�����ⶨ���ƫ�ͣ�������ɸý������Ҫԭ���� ��
��5��������Ŀ���������ݼ���������ܵ�����Ϊ %��������������С�����1λ����


