��Ŀ����
��һ���������ܱ������м���2mol A��0.6mol C��һ������B�������塣һ�������·�����Ӧ��������Ũ����ʱ��仯��ͼһ��ʾ��ͼ��Ϊt2ʱ�̺�ı䷴Ӧ������ƽ����ϵ�з�Ӧ������ʱ��仯����������ĸ��ζ����ı�һ�ֲ�ͬ����������֪t3��t4��Ϊʹ�ô�����ͼһ��t0��t1��c��B��δ������
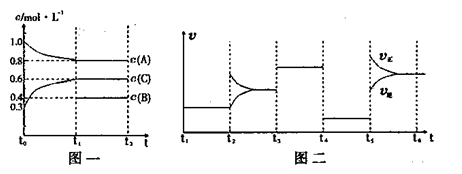
����˵������ȷ���ǣ� ��
A�����¶��¸÷�Ӧ�Ļ�ѧ����ʽ2A��g��+B��g�� 2C��g��
2C��g��
B��t4��t5�θı������Ϊ��Сѹǿ
C��B����ʼ���ʵ���Ϊ1.0mol
D������ͬ�����£�����ʼʱ�����м���a mol A��bmol B��cmol C��Ҫ�ﵽt1ʱ��ͬ����ƽ�⣬a��b��cҪ���������Ϊa+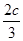 ��2.4 �� b+
��2.4 �� b+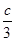 ��1.2
��1.2

����˵������ȷ���ǣ� ��
A�����¶��¸÷�Ӧ�Ļ�ѧ����ʽ2A��g��+B��g��
 2C��g��
2C��g��B��t4��t5�θı������Ϊ��Сѹǿ
C��B����ʼ���ʵ���Ϊ1.0mol
D������ͬ�����£�����ʼʱ�����м���a mol A��bmol B��cmol C��Ҫ�ﵽt1ʱ��ͬ����ƽ�⣬a��b��cҪ���������Ϊa+
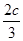 ��2.4 �� b+
��2.4 �� b+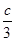 ��1.2
��1.2A
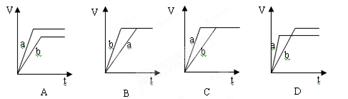
������ͼ�����ʽ����Ӱ�컯ѧ��Ӧ�����Լ���ѧƽ���ƶ������ء�ͼ���������ֲ�ͬ�����Ӱ�컯ѧ��ƽ�⣬��Ӱ��ƽ��������У�Ũ�ȡ��¶ȡ�ѹǿ��������t3��t4��t4��t5������ƽ���Dz��ƶ��ģ���ֻ����ѹǿ�ʹ���Ӱ��ģ����Ӧ���ƶϸ÷�ӦΪ������仯�ķ�Ӧ���ٽ��ͼһ��֪��A�ı仯Ϊ0.2mol/L��C�ı仯��Ϊ0.3mol/L���ɹ���֪����ѧ��Ӧ������֮�ȵ��ڻ�ѧ����ʽǰ�ļ���ϵ���ȣ���д���÷�Ӧ�ķ���ʽΪ2A(g)+B(g) 3C(g)����A����ȷ��t3��t4��ƽ���ԭƽ�������Ҫ�죬��t4��t5�������ֱ�������ǰ��Ӧ�ǼӴ���������Ϊ��ѹ����Ϊ����ֻ����һ�Σ���B����ȷ��B��ƽ��Ũ����0.4mol/L������ʼʱӦΪ0.5mol/L����ʼ2molA����Ӧ��Ũ��Ϊ1mol/L�������Ӧ��2L������B����ʼ���ʵ���Ϊ0.5mol/L��2L��1mol��C����ȷ�� D�����ǵ�Чƽ�⡣Ҫ����Ϊ��ͬƽ�⣬������������ԭƽ����ȡ�
3C(g)����A����ȷ��t3��t4��ƽ���ԭƽ�������Ҫ�죬��t4��t5�������ֱ�������ǰ��Ӧ�ǼӴ���������Ϊ��ѹ����Ϊ����ֻ����һ�Σ���B����ȷ��B��ƽ��Ũ����0.4mol/L������ʼʱӦΪ0.5mol/L����ʼ2molA����Ӧ��Ũ��Ϊ1mol/L�������Ӧ��2L������B����ʼ���ʵ���Ϊ0.5mol/L��2L��1mol��C����ȷ�� D�����ǵ�Чƽ�⡣Ҫ����Ϊ��ͬƽ�⣬������������ԭƽ����ȡ�
 3C(g)����A����ȷ��t3��t4��ƽ���ԭƽ�������Ҫ�죬��t4��t5�������ֱ�������ǰ��Ӧ�ǼӴ���������Ϊ��ѹ����Ϊ����ֻ����һ�Σ���B����ȷ��B��ƽ��Ũ����0.4mol/L������ʼʱӦΪ0.5mol/L����ʼ2molA����Ӧ��Ũ��Ϊ1mol/L�������Ӧ��2L������B����ʼ���ʵ���Ϊ0.5mol/L��2L��1mol��C����ȷ�� D�����ǵ�Чƽ�⡣Ҫ����Ϊ��ͬƽ�⣬������������ԭƽ����ȡ�
3C(g)����A����ȷ��t3��t4��ƽ���ԭƽ�������Ҫ�죬��t4��t5�������ֱ�������ǰ��Ӧ�ǼӴ���������Ϊ��ѹ����Ϊ����ֻ����һ�Σ���B����ȷ��B��ƽ��Ũ����0.4mol/L������ʼʱӦΪ0.5mol/L����ʼ2molA����Ӧ��Ũ��Ϊ1mol/L�������Ӧ��2L������B����ʼ���ʵ���Ϊ0.5mol/L��2L��1mol��C����ȷ�� D�����ǵ�Чƽ�⡣Ҫ����Ϊ��ͬƽ�⣬������������ԭƽ����ȡ�
��ϰ��ϵ�д�
 �����ҵ��ٿ���������������ϵ�д�
�����ҵ��ٿ���������������ϵ�д�
�����Ŀ
 N2O4��g�� ��H��0������ƿ
N2O4��g�� ��H��0������ƿ N2O4,���NO2ת����Ϊa%�����¶ȡ��������ʱ,��ͨ��1mol NO2,�����½���ƽ��ʱ,���NO2ת����Ϊb%,a��b�Ƚϣ� ��
N2O4,���NO2ת����Ϊa%�����¶ȡ��������ʱ,��ͨ��1mol NO2,�����½���ƽ��ʱ,���NO2ת����Ϊb%,a��b�Ƚϣ� �� C(g)���ܱ������н��У���������У�����ʹ��ѧ��Ӧ���ʼӿ����
C(g)���ܱ������н��У���������У�����ʹ��ѧ��Ӧ���ʼӿ����  ��Ӧ��ȡ
��Ӧ��ȡ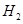 ʱ�����д�ʩ����ʹ
ʱ�����д�ʩ����ʹ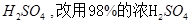
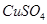
 2SO3(g)����H<0������Ӧ������ʱ��ı仯������Ը��ݴ������ж�˵��������ȷ����(����)
2SO3(g)����H<0������Ӧ������ʱ��ı仯������Ը��ݴ������ж�˵��������ȷ����(����)