��Ŀ����
�����Ƶ���;�dz��㷺������ɱ������ɱ�����ľ�ķ�������ʵ���ҿ�ͨ����ͼ��ʾ����������ȡ

����ÿС��������Ϣ���ش������й����⣺
(1)��֪�����ͷ�����(H2SiF6)��ҺΪ��ɫ���ķ���Һ�壬�ܶ�Ϊ1.32 g/mL���ӷ����д̼�����ζ��������ʵ���Ũ�ȵ�H2SO4�����൱�����Բ������մɶ��н�ǿ�ĸ�ʴ�ԡ�����ʵ����������ȫ���þ��������(�磺 )���ɣ��ڸ���������ɵ������л��NH4HCO3���塢H2SiF6Ũ��Һ��ˮʱ�������Լ���˳��Ӧ��________________ ���Ӻ��Լ���Ϊ�������dz�ַ�Ӧ��������
)���ɣ��ڸ���������ɵ������л��NH4HCO3���塢H2SiF6Ũ��Һ��ˮʱ�������Լ���˳��Ӧ��________________ ���Ӻ��Լ���Ϊ�������dz�ַ�Ӧ��������
_______ �����裨����ĸ����
A������ B���� C�������� D���մ�
(2)��֪��20��ʱNa2SiF6���ܽ��Ϊ2.12 g����д��1 L 1 mol/L��H2SiF6��Һ��1 L 1 mol/LNa2CO3��Һ��Ϻ�����Һ��ˮ������Լ����2000 g��������ѧ��Ӧ�����ӷ���ʽΪ__________________��
(3)��������ͼ�в�������Һ��ԭ����������___������ĸ����
A��NH4F B��H2SiO3 C��(NH4)2SiF6 D��(NH4)2CO3
(4)����1���Ƿ���Ҫ�Գ�������ϴ�ӣ�____����ǡ�����ԭ����__________________��
(5)��ҺB�о�����________________����������ƣ���ͬ���ɵõ�����Ʒ�Ȼ�泥��ֲ�Ʒ���徭______�ɵô����IJ�Ʒ��
(1)��֪�����ͷ�����(H2SiF6)��ҺΪ��ɫ���ķ���Һ�壬�ܶ�Ϊ1.32 g/mL���ӷ����д̼�����ζ��������ʵ���Ũ�ȵ�H2SO4�����൱�����Բ������մɶ��н�ǿ�ĸ�ʴ�ԡ�����ʵ����������ȫ���þ��������(�磺
 )���ɣ��ڸ���������ɵ������л��NH4HCO3���塢H2SiF6Ũ��Һ��ˮʱ�������Լ���˳��Ӧ��________________ ���Ӻ��Լ���Ϊ�������dz�ַ�Ӧ��������
)���ɣ��ڸ���������ɵ������л��NH4HCO3���塢H2SiF6Ũ��Һ��ˮʱ�������Լ���˳��Ӧ��________________ ���Ӻ��Լ���Ϊ�������dz�ַ�Ӧ��������_______ �����裨����ĸ����
A������ B���� C�������� D���մ�
(2)��֪��20��ʱNa2SiF6���ܽ��Ϊ2.12 g����д��1 L 1 mol/L��H2SiF6��Һ��1 L 1 mol/LNa2CO3��Һ��Ϻ�����Һ��ˮ������Լ����2000 g��������ѧ��Ӧ�����ӷ���ʽΪ__________________��
(3)��������ͼ�в�������Һ��ԭ����������___������ĸ����
A��NH4F B��H2SiO3 C��(NH4)2SiF6 D��(NH4)2CO3
(4)����1���Ƿ���Ҫ�Գ�������ϴ�ӣ�____����ǡ�����ԭ����__________________��
(5)��ҺB�о�����________________����������ƣ���ͬ���ɵõ�����Ʒ�Ȼ�泥��ֲ�Ʒ���徭______�ɵô����IJ�Ʒ��
(1)NH4HCO3���塢ˮ��H2SiF6Ũ��Һ��ABD
(2)2H++SiF62-+2Na++CO32-=Na2SiF6��+CO2��+H2O
(3)B
(4)�ǣ�ʹNH4F���������������ģ�ȫ��ת�Ƶ���ҺA�У�����߲�Ʒ���ʵ�˵�����ɣ�
(5)������Ũ����������ȴ���ᾧ���ؽᾧ
(2)2H++SiF62-+2Na++CO32-=Na2SiF6��+CO2��+H2O
(3)B
(4)�ǣ�ʹNH4F���������������ģ�ȫ��ת�Ƶ���ҺA�У�����߲�Ʒ���ʵ�˵�����ɣ�
(5)������Ũ����������ȴ���ᾧ���ؽᾧ

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ

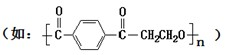 ���ɣ��ڸ���������ɵ������л��NH4HCO3���塢H2SiF6Ũ��Һ��H2Oʱ�������Լ���˳��Ӧ��
���ɣ��ڸ���������ɵ������л��NH4HCO3���塢H2SiF6Ũ��Һ��H2Oʱ�������Լ���˳��Ӧ��
 ���ɣ��ڸ���������ɵ������л��NH4HCO3���塢H2SiF6Ũ��Һ��H2Oʱ�������Լ���˳��Ӧ��______���Ӻ��Լ���Ϊ�������dz�ַ�Ӧ��������______�����裨����ĸ����
���ɣ��ڸ���������ɵ������л��NH4HCO3���塢H2SiF6Ũ��Һ��H2Oʱ�������Լ���˳��Ӧ��______���Ӻ��Լ���Ϊ�������dz�ַ�Ӧ��������______�����裨����ĸ����


 ���ɣ��ڸ���������ɵ������л��NH4HCO3���塢H2SiF6Ũ��Һ��H2Oʱ�������Լ���˳��Ӧ�� ���Ӻ��Լ���Ϊ�������dz�ַ�Ӧ�������� �����裨����ĸ����
���ɣ��ڸ���������ɵ������л��NH4HCO3���塢H2SiF6Ũ��Һ��H2Oʱ�������Լ���˳��Ӧ�� ���Ӻ��Լ���Ϊ�������dz�ַ�Ӧ�������� �����裨����ĸ����