��Ŀ����
��10�֣���1����ָ�����������зֱ�Ӧ������Щ���뷽���������ˡ����������� ����ȡ���ᾧ��
a��������ҩ__________________ b�����붹���Ͷ���__________________
c���ú�ˮɹ��_________________ d����ˮ����__________________
(2 )����һ������ˮ��Һ��ֻ���ܺ������������е������֣�K����H����M g2����Ba2����CO32����SO42������ȡ����100mL��Һ��������ʵ�飺
g2����Ba2����CO32����SO42������ȡ����100mL��Һ��������ʵ�飺
�ٵ�һ�ݼ�������NaHCO3��Һ���ռ�������0.03mol��
�ڵڶ��ݼ�����Ba(NO3)2��Һ��ַ�Ӧ����˸���ø������4.66g��
��������ʵ��ش�
��a ��ԭ��Һ��һ�������ڵ�������_______________________��
��ԭ��Һ��һ�������ڵ�������_______________________��
��b��ԭ��Һ�п��ܴ��ڵ�������_______________________��
��c��������ԭ��Һ��һ�����ڵ������ӵ����ʵ���Ũ��________________��
a��������ҩ__________________ b�����붹���Ͷ���__________________
c���ú�ˮɹ��_________________ d����ˮ����__________________
(2 )����һ������ˮ��Һ��ֻ���ܺ������������е������֣�K����H����M
 g2����Ba2����CO32����SO42������ȡ����100mL��Һ��������ʵ�飺
g2����Ba2����CO32����SO42������ȡ����100mL��Һ��������ʵ�飺�ٵ�һ�ݼ�������NaHCO3��Һ���ռ�������0.03mol��
�ڵڶ��ݼ�����Ba(NO3)2��Һ��ַ�Ӧ����˸���ø������4.66g��
��������ʵ��ش�
��a
 ��ԭ��Һ��һ�������ڵ�������_______________________��
��ԭ��Һ��һ�������ڵ�������_______________________����b��ԭ��Һ�п��ܴ��ڵ�������_______________________��
��c��������ԭ��Һ��һ�����ڵ������ӵ����ʵ���Ũ��________________��
( 10��)
�� 1 ��a ��ȡ b ���� c ���� d ���� ( 4��)
�� 2 ��( a ) Ba2+ CO32- ( b )K����Mg2�� (c )0.2 mol/L (6��)
�� 1 ��a ��ȡ b ���� c ���� d ���� ( 4��)
�� 2 ��( a ) Ba2+ CO32- ( b )K����Mg2�� (c )0.2 mol/L (6��)
��

��ϰ��ϵ�д�
 ǧ�������������ĩ�����Ծ�����ϵ�д�
ǧ�������������ĩ�����Ծ�����ϵ�д�
�����Ŀ
 ����ѧϰС������˽���MnO2�����н϶��MnO��MnCO3����Ʒת��Ϊ��MnO2��ʵ�飬���������£�
����ѧϰС������˽���MnO2�����н϶��MnO��MnCO3����Ʒת��Ϊ��MnO2��ʵ�飬���������£�
 ����Ӧ�����ӷ���ʽΪ��5Mn2+ + 2ClO3- + 4H2O �� 5MnO2�� + Cl2��
����Ӧ�����ӷ���ʽΪ��5Mn2+ + 2ClO3- + 4H2O �� 5MnO2�� + Cl2�� + 8H+
+ 8H+ ��
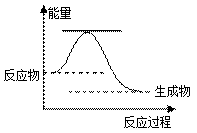
��
 ����֬������Ӧ�Ļ��Һ�з������֬������________��
����֬������Ӧ�Ļ��Һ�з������֬������________�� ����________��
����________�� aCl________��
aCl________�� ����Ҫʱ���Լ��ȣ�
����Ҫʱ���Լ��ȣ�
 ��������)
��������)