��Ŀ����
12����֪�����³�ѹ�£�D��E��F��I��JΪ���壻E��ʹ����ʯ��ˮ����ǣ�1molE�뺬1molF��ˮ��Һǡ�÷�Ӧ����B��B��һ�ֳ����Ļ��ʣ�����A-K֮��������ͼ��ʾת����ϵ�����ַ�Ӧ�����ɵ�ˮ����ȥ����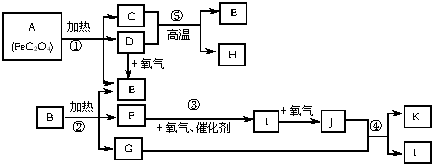
�Իش��������⣺
��1����֪A��[FeC2O4]��C��FeԪ�ؾ�Ϊ+2�ۣ���FeC2O4��CԪ�صĻ��ϼ�Ϊ+3��E�Ļ�ѧʽΪCO2��
��2��д����Ӧ�ۡ��ܡ��ݵĻ�ѧ����ʽ
��4NH3+5O2 $\frac{\underline{����}}{��}$4NO+6H2O��3NO2+H2O=2HNO3+NO��FeO+CO$\frac{\underline{\;����\;}}{\;}$Fe+CO2
��3������H��K��Ũ��Һ��һ�������·�Ӧ����1mol K����ԭʱ��ת�Ƶĵ�������Ϊ6.02��1023����
��4��д����������H��K��ϡ��Һ��Ӧ�����ӷ���ʽ��3Fe+2NO3-+8H+=3Fe2++2NO��+4H2O��
���� A��[FeC2O4]��C��FeԪ�ؾ�Ϊ+2�ۣ�A���ȷֽ�õ�C������D������E����D��������Ӧ�õ�E������֪CΪFeO��DΪCO��EΪCO2���ɷ�Ӧ�ݿ�֪HΪFe��B��һ�ֳ����Ļ��ʣ����ȷֽ�õ�������̼������F��G����F��������������Ӧ��BΪ̼���γɵ���Σ�1molE�뺬1molF��ˮ��Һǡ�÷�Ӧ����B��BΪNH4HCO3��FΪNH3��IΪNO��JΪNO2��GΪH2O��KΪHNO3���ݴ˽��
��� �⣺A��[FeC2O4]��C��FeԪ�ؾ�Ϊ+2�ۣ�A���ȷֽ�õ�C������D������E����D��������Ӧ�õ�E������֪CΪFeO��DΪCO��EΪCO2���ɷ�Ӧ�ݿ�֪HΪFe��B��һ�ֳ����Ļ��ʣ����ȷֽ�õ�������̼������F��G����F��������������Ӧ��BΪ̼���γɵ���Σ�1molE�뺬1molF��ˮ��Һǡ�÷�Ӧ����B��BΪNH4HCO3��FΪNH3��IΪNO��JΪNO2��GΪH2O��KΪHNO3��
��1��A��[FeC2O4]��C��FeԪ�ؾ�Ϊ+2�ۣ���FeC2O4��̼Ԫ�صĻ��ϼ�Ϊ+3��E�Ļ�ѧʽΪCO2��
�ʴ�Ϊ��+3��CO2��
��2����Ӧ�۵Ļ�ѧ����ʽΪ��4NH3+5O2 $\frac{\underline{����}}{��}$4NO+6H2O��
��Ӧ�ܵĻ�ѧ����ʽΪ��3NO2+H2O=2HNO3+NO��
��Ӧ�ݵĻ�ѧ����ʽΪ��FeO+CO$\frac{\underline{\;����\;}}{\;}$Fe+CO2��
�ʴ�Ϊ��4NH3+5O2 $\frac{\underline{����}}{��}$4NO+6H2O��3NO2+H2O=2HNO3+NO��FeO+CO$\frac{\underline{\;����\;}}{\;}$Fe+CO2��
��3������Fe��HNO3��Ũ��Һ��Ӧ�����������������������һ�������·�Ӧ����1mol HNO3����ԭʱ��ת�Ƶĵ�������Ϊ1mol����6-4����6.02��1023mol-1=6.02��1023��
�ʴ�Ϊ��6.02��1023��
��4������Fe��HNO3��ϡ��Һ��Ӧ�����ӷ���ʽ��3Fe+2NO3-+8H+=3Fe2++2NO��+4H2O���ʴ�Ϊ��3Fe+2NO3-+8H+=3Fe2++2NO��+4H2O��
���� ���⿼�������ƶϣ����ضԻ�ѧ����Ŀ��飬��Ҫѧ����������Ԫ�ػ�����֪ʶ���Ѷ��еȣ�

 ����ѧ����ϵ�д�
����ѧ����ϵ�д�| A�� | Na��Al��Fe����������һ����������ˮ��Ӧ������H2�Ͷ�Ӧ�ļ� | |
| B�� | �����ۼ���FeCl3��CuCl2�����Һ�У���ַ�Ӧ��ʣ��Ĺ����б���ͭ | |
| C�� | ��SO2ͨ��Ca��ClO��2��Һ������CaSO3���� | |
| D�� | ������ͭ��Ũ���ᷴӦ�����ɵ�����ֻ��NO2 |
| A�� | ̼��������Һ�м�������CO32-+2H+�TCO2��+H2O | |
| B�� | �����������ᷴӦS2-+2H+�TH2S�� | |
| C�� | ���������ڴ�����ҺC6H5O-+CH3COOH��C6H5OH+CH3COO- | |
| D�� | �Ȼ�������Һ��ͨ����������Fe2++Cl2�TFe3++2Cl- |
��1���������Һ�и������ӵ�Ũ�ȣ�����Ӧ�������±���
| c ��H+�� | c��SO42-�� | c ��Fe2+��Fe3+�� |
��2��ԭ��Һ��Fe2+�������İٷ��ʣ���������Fe2+���ʵ���ռFe2+��Fe3+�����ʵ����İٷֱȣ�20%��
��3����Fe2+���ֱ�����ʱ���Ƶ�c��Fe2+��Fe3+����c��H+����c��SO42-���Ĺ�ϵ��c��SO42-��-$\frac{1}{2}$c��H+����c��Fe2+��Fe3+����$\frac{2}{3}$c��SO42-��-$\frac{1}{3}$c��H+����
| A�� | 22.4LCO��CO2�Ļ��������������̼ԭ����һ����NA | |
| B�� | ���³�ѹ�£�22.4L����������þ�۳�ַ�Ӧ��ת�Ƶĵ�����Ϊ2NA | |
| C�� | ���³�ѹ�£�32gO2��32gO3������ԭ��������2NA | |
| D�� | 1L1mol/L��������Һ�к�HCl�ķ�����ΪNA |
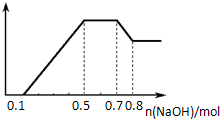
| A�� | ��Һ�е���������H+��Mg2+��Al3+��NH4+ | |
| B�� | ��Һ��n��NH4+��=0.2 mol | |
| C�� | ��Һ��һ������CO32-��NO3-�����ܺ���SO42- | |
| D�� | n��H+����n��Al3+����n��Mg2+��=2��2��1 |
 ��
��