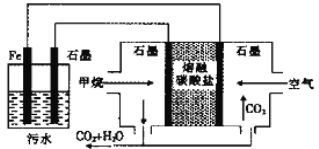
��Ŀ����
����Ŀ����Ϊ�˴ﵽ�±��е�ʵ��Ҫ����ӡ���ѡ��Ļ�ѧ�Լ������������У�ѡ����ȷѡ�����ĸ���������Ӧ�Ŀո��С�
��� | ʵ��Ҫ�� | �� | ��ѡ��Ļ�ѧ�Լ������� |
a | ���������Ƿ�������� | ____ | A�����Ƶ�������ͭ����Һ |
b | ����ֲ�������Ƿ���̼̼˫�� | ____ | B����ɫʯ����Һ |
c | ������Һ���Ƿ��������� | ____ | C�����뱥��Na2CO3��Һ����Һ |
d | ��ȥ���������е��������� | ____ | D����ˮ |
��A�Ľṹ��ʽ���£�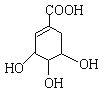
��1��A���ӵķ���ʽΪ��___________��
��2��A���������ֺ��������ŵ�������_____��______��
��3����д��A�����������Ľ����Ʒ�����Ӧ�Ļ�ѧ����ʽ____________��
III ��̼���⡢��3��Ԫ����ɵ��л���A����Է�������Ϊ102��������������� Ϊ9.8%����������ԭ�Ӹ���Ϊ��ԭ�Ӹ�����5����
��1��A�ķ���ʽ��____________��
��2��A��NaHCO3��Һ��Ӧ��ͬ���칹����_____________ �֡�
��3��A������һ������ͬ���칹�壬���칹��������������ˮ�⣬����������Է���������ͬ�Ļ����д�����ϸ����������Ľṹ��ʽ��2�֣���_________________��________________��
���𰸡� B D A C C7H10O5 �ǻ� �Ȼ� �� C5H10O2 4 CH3COOCH��CH3��2 CH3COOCH2CH2CH3
������������a������������ԣ���ʹ���ָʾ����ɫ��������ɫʯ����Һ���飬��Һ���ɫ���ʴ�ΪB��b��̼̼˫��������ˮ�����ӳɷ�Ӧ����ʹ��ˮ��ɫ���ʴ�ΪD��c�������Ǻ���ȩ��������������ͭ��Һ����������ԭ��Ӧ���ڼ��������½��У�����ש��ɫ�������ʴ�ΪA��d���������������ڱ���̼������Һ��������̼���Ʒ�Ӧ�������ڱ���̼������Һ���飬�ʴ�ΪC��
����(1)(1)A���ӵķ���ʽΪC7H10O5��
(2)�л��ﺬ���ǻ����Ȼ���̼̼˫����
(3)�����Ʒ�Ӧ������������Ӧ�ķ���ʽΪ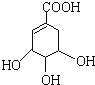 +4Na��
+4Na��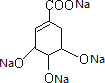 +2H2����
+2H2����
III��(1)�л���A����Է���������102���������������Ϊ9.8%��������к��е�N(H)= ![]() =10����������ԭ�Ӹ���Ϊ��������5����N(O)�T2��������Է���������102������N(C)=5�������л���A�ķ���ʽΪC5H10O2��
=10����������ԭ�Ӹ���Ϊ��������5����N(O)�T2��������Է���������102������N(C)=5�������л���A�ķ���ʽΪC5H10O2��
(2)A��NaHCO3��Һ��Ӧ˵��������������Ȼ��������������AΪCH3CH2CH2CH2COOH��CH3CH(CH3)CH2COOH��CH3CH2CH(CH3)COOH��CH3C(CH3)2COOH����4�֣�
(3)A��һ������ͬ���칹�壬������������ˮ�⣬����������Է���������ͬ�Ļ�������������Ľṹ��ʽΪ��CH3COOCH(CH3)2��CH3COOCH2CH2CH3��

 ǧ�������������ĩ�����Ծ�����ϵ�д�
ǧ�������������ĩ�����Ծ�����ϵ�д�