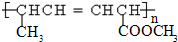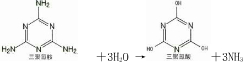��Ŀ����
ʳƷ��ȫ��ϵ���������������ܹ�ע��
��ɽ���ᣨCH3CH=CHCH=CHCOOH���Ǹ�Ч��ȫ��ʳƷ��������
��1�����й���ɽ����������������ȷ����__________
A.������ˮ�����ӳɷ�Ӧ B.��ʹ���Ը��������ɫ
C.��������ϵ��ˮ�� D.�ܷ����Ӿ۷�Ӧ
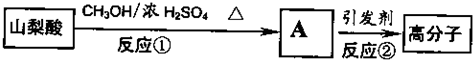
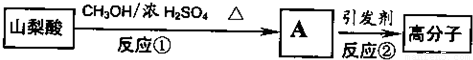
��2��ɽ���ỹ���Ժϳ���������Ȼ�ĸ߷��Ӳ��ϣ��ϳ�·�����£�

д���ٵķ�Ӧ����ʽ��________________________;�ڵķ�Ӧ���ͣ�_________
����¹�̷����������谷����Ӥ���ش��¹���������ش��������⣺
��1���̷��е�����ƽ��������Ϊ16%�������谷�е�����������Ϊ______
��2�������谷�������ڷ���ȡ����Ӧ��ˮ�⣩�������������ᣨ��ͼ����

д�������谷ˮ�ⷽ��ʽ______________________
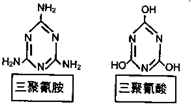
��3��������ҵ��ԭ������������CO(NH2)2�������¿������������谷��NH3��CO2���̷��в��������ˣ����Ǻ�������Ȼ�ϸߡ�д�����ظ��²��������谷�Ļ�ѧ����ʽ________
��1��C(2��)
��2��CH3CH=CHCH=CHCOOH �� CH3OH ![]() CH3CH=CHCH=CHCOOCH3+ H2O��2�֣�
CH3CH=CHCH=CHCOOCH3+ H2O��2�֣�
�Ӿ۷�Ӧ(1��)
��1�� 66.7%(1��)
��2��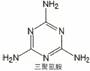 ��3H2O ��
��3H2O �� ��3NH3 (2��)
��3NH3 (2��)
��3��6 CO��NH2)2 �� C3N6H6 + 6 NH3 + 3 CO2(2��)