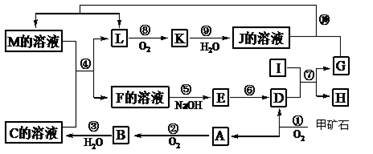
��Ŀ����
���п�ͼ�е���ĸ�ֱ����һ�ֳ��������ʻ�����Һ���֮���ת����ϵ����ͼ��ʾ�����ֲ��P��Ӧ��������ȥ������֪A��BΪ��̬���ʣ�F�ǵؿ��к������Ľ���Ԫ�صĵ��ʣ�E��H��IΪ�����EΪ��ɫ���壬IΪ����ɫ���壻MΪ���ɫ������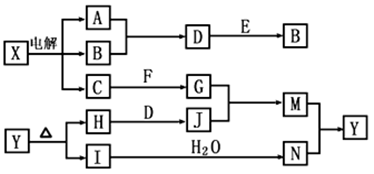
��ش��������⣺
��1��B������Ԫ��λ�����ڱ��е� ���ڣ� �塣
��2��A��B��ȼ�յ������� ��
��3��D+E��B�ķ�Ӧ�У��������뱻��ԭ�����ʵ����ʵ������� ��
��4��G+J��M�����ӷ���ʽ�� ��
��5��Y���ȷֽ�Ļ�ѧ����ʽ�� ��
��1���� VIIA
��2��������ɫ����
��3��2:1
��4��3AlO2��+Fe3��+6H2O=3Al(OH)3��+Fe(OH)3��
��5��4Fe(NO3)3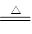 2Fe2O3+12NO2��+3O2��
2Fe2O3+12NO2��+3O2��
����

��ϰ��ϵ�д�
 ͨ��ѧ��Ĭд����ϵ�д�
ͨ��ѧ��Ĭд����ϵ�д� ���ƽ̸�������ѡ����ĩ���100��ϵ�д�
���ƽ̸�������ѡ����ĩ���100��ϵ�д�
�����Ŀ