��Ŀ����
(13��)��֪������Aǿ�ȿɵõ�B��C��D��E�������ʣ�Bͨ�������Ϊ��ɫ��ζҺ�壬E��F �ǿ�����Ҫ�ɷ֣�D�ܲ������꣬IΪ����ɫ���壬C��J��Ӧ�ɵ�A��J��KΪ���ֳ������ᡣ����֮���ת����ϵ��ͼ��ʾ(ͼ�в��ַ�Ӧ��������P��Ӧ����δ�г����� 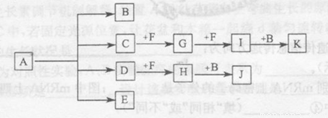
��ش��������⣺
(1)E���ʵĵ���ʽ��________��
(2)����C����ֽ��________������D���Լ���________(����ֽ���Լ�����)��
(3)д��Aǿ�ȷֽ�����B��C��D��E�Ļ�ѧ����ʽ________��
(4)д��Dͨ��FeCl3��Һʱ��������Ӧ�����ӷ���ʽ_____ ��
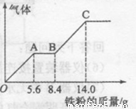
(5) ����Ũ��J��K��Ϻ��ϡ��Һ200mL��ƽ���ֳ����ݡ�������һ��������ͭ�ۣ�������ܽ�a g(��������ֻΪG)������һ�����������ۣ�������������������������ӵı仯��ͼ��ʾ�����a��________g��������G��״�������Ϊ________����J�����ʵ���Ũ��Ϊ______��
��13�֣���1�� ��2�֣� ��2����ɫʯ����ֽ Ʒ����Һ ��2�֣�
��2�֣� ��2����ɫʯ����ֽ Ʒ����Һ ��2�֣�
��3��3(NH4)2SO4 6H2O��4NH3����3SO2����N2�� ��3�֣�
6H2O��4NH3����3SO2����N2�� ��3�֣�
��4��SO2��2Fe3����2H2O��SO42����2Fe2����4H�� ��3�֣�
��5����9.6g ��1�֣� ��2.24L ��1�֣� ��2.5mol/L ��1�֣�
��������
���������Bͨ�������Ϊ��ɫ��ζҺ�壬���BӦ����ˮ��E��F �ǿ�����Ҫ�ɷ֣�������ǵ�����������D�ܲ������꣬����D��SO2��SO2�ܺ�F��Ӧ����H����F��������E�ǵ�����H�������������������ˮ��Ӧ����J��J�����ᡣIΪ����ɫ���壬��I��NO2��G��������Ӧ����NO2������G��NO��NO2����ˮ���������NO��K���ᣬ��K�����ᡣC��������Ӧ����NO����C��J��Ӧ�ɵ�A������C�ǰ�����A��������李�
��1�������Ǻ��зǼ��Լ��ĵ��ʣ������ʽ�� ��
��
��2�������Ǽ������壬���ú�ɫʯ����ֽ���飻SO2����Ư���ԣ�����Ʒ����Һ���顣
��3��Aǿ�ȷֽ�����B��C��D��E�Ļ�ѧ����ʽ3(NH4)2SO4 6H2O��4NH3����3SO2����N2����
6H2O��4NH3����3SO2����N2����
��4��SO2���л�ԭ�ԣ��ܱ���������������˸÷�Ӧ�����ӷ���ʽ��SO2��2Fe3����2H2O��SO42����2Fe2����4H����
��5���������������ᣬ���Ը���ͼ���֪��O��A�����ķ�Ӧ��Fe��4H����NO3����Fe3����NO����2H2O��A��B������Ӧ�ķ���ʽ��2Fe3����Fe��3Fe2����B��C������Ӧ�ķ���ʽ��Fe��2H����Fe2����H2���������������������ʵ����ֱ���5.6g��56g/mol��0.1mol����8.4g��5.6g����56g/mol��0.05mol����14.0g��8.4g����56g/mol��0.1mol�����Ը��ݵ�Ԫ���غ��֪����������ʵ�����0.1mol��������Һ��ֻ�����������������ԭ���غ��֪���������������ʵ�����0.25mol�������������ʵ���Ҳ��0.25mol���������Ũ����0.25mol��0.1L��2.5mol/L����һ����Һ�У������Ӻ�NO3�������ʵ����ֱ���0.6mol��0.1mol������ݷ���ʽ3Cu��8H����2NO3����3Cu2����2NO����4H2O��֪�������ӹ���������NO3����ȫ����ԭ����NO����NO�����ʵ�����0.1mol���ڱ�״���µ������2.24L�������ܽ�ͭ��������0.15mol��64g/mol��9.6g��
���㣺���������ƶϣ�������飻���������Ļ��Һ�������Ӧ�ļ����
