��Ŀ����
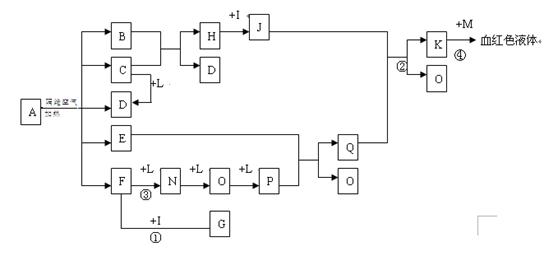
��14�֣���֪AΪһ���Σ�C��D��F��N��OΪ��ɫ���壬E���³�ѹ��Ϊ��ɫ��ζ��Һ�壬N��H��LΪ���г����ĵ��ʣ�IΪ��������ǿ�ᣬM����ɫ��ӦΪ��ɫ����Ӧ�ٳ���������F�ļ��顣
��1��д��G�ĵ���ʽ_______________��M�Ļ�ѧʽ_____________��
��2��д����Ӧ�ڵ����ӷ�Ӧ����ʽ______________________________��
��3��д����Ӧ�۵Ļ�ѧ��Ӧ����ʽ_____________����Ӧ1-4�����ڷ�������ԭ��Ӧ���� ��
��4����ʯī�缫�����ҺKʱ������ʼ�ε缫��Ӧ����ʽΪ��
����___________________
����___________________
��5����֪A�ڸ������������·ֽ�����ĸ���������ʵ���֮��ΪB��C��D��E��F=1��2��2��2��2��д��A�ֽ�ķ�Ӧ����ʽ__________________________
��14����
��1�� ��2�֣� KSCN ��1�֣�
��2�֣� KSCN ��1�֣�
��2��3Fe2++NO3-+4H+= 3Fe3++NO��+2H2O ��2�֣�
��3��4NH3+3O2=2N2+6H2O ��2��14��2�֣�
��4��������Fe3++e= Fe2+ ��1�֣� ������2Cl- - 2e= Cl2 �� ��1�֣�
��5��(NH4) 2Fe(C2O4)2=FeO+2CO��+2CO2��+2H2O+2NH3 �� ��3�֣�
��������

 �ִʾ��ƪϵ�д�
�ִʾ��ƪϵ�д�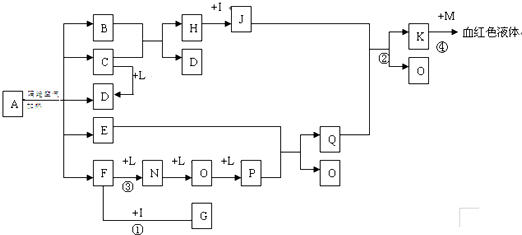
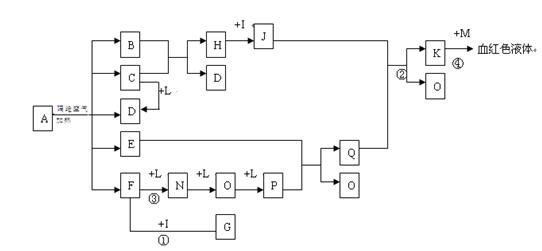
 ��D��F��N��OΪ��ɫ���壬E���³�ѹ��Ϊ��ɫ��ζ��Һ�壬N��H��LΪ���г����ĵ��ʣ�IΪ��������ǿ�ᣬM����ɫ��ӦΪ��ɫ����Ӧ�ٳ���������F�ļ��顣
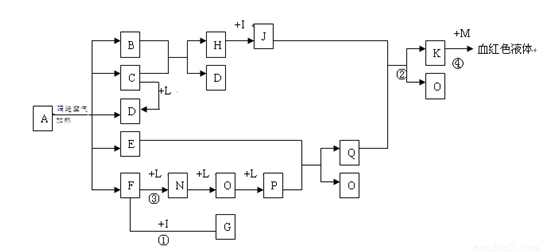
��D��F��N��OΪ��ɫ���壬E���³�ѹ��Ϊ��ɫ��ζ��Һ�壬N��H��LΪ���г����ĵ��ʣ�IΪ��������ǿ�ᣬM����ɫ��ӦΪ��ɫ����Ӧ�ٳ���������F�ļ��顣