��Ŀ����
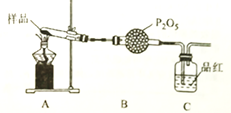
����Ŀ������ͼ��ʾ��C��D��E��F��X��Y���Ƕ��Ե缫������Դ��ͨ�������е����̪��Һ����F�������Ժ�ɫ��������������⣺

��1����ԴB����������________��
��2����װ���е�ⷴӦ���ܻ�ѧ����ʽ��________________________��
��3������ռ���װ�������������������ʣ��������ʵ����ʵ���������������������________��
��4�����ñ�װ�ø�ͭ������GӦ����____������ͭ�����������������Һ����Ҫ�ɷ���______���ѧʽ����
���𰸡� ���� 2NaCl+2H2O![]() H2 �� +Cl2 �� + 2NaOH 1:2 �� AgNO3
H2 �� +Cl2 �� + 2NaOH 1:2 �� AgNO3
���������������������Դ��ͨ�������е����̪��Һ����F�������Ժ�ɫ��˵��F���Լ��ԣ���F����ӦΪ![]() ��F�����������Ե�ԴB�Ǹ�����A��������
��F�����������Ե�ԴB�Ǹ�����A��������
�������������Ϸ�������1����ԴB���������Ǹ�����
��2���ö��Ե缫����Ȼ�����Һ��������������������������������װ���е�ⷴӦ���ܻ�ѧ����ʽ��2NaCl+2H2O![]() H2 �� +Cl2 �� + 2NaOH��
H2 �� +Cl2 �� + 2NaOH��
��3����װ��C����ӦʽΪ![]() ,D���ļ���ӦʽΪ
,D���ļ���ӦʽΪ![]() �����ݵ����غ㣬�������ʵ����ʵ�������1:2��
�����ݵ����غ㣬�������ʵ����ʵ�������1:2��
��4�����ʱ�Ƽ����������Ʋ���������������жƲ����������Һ������ʣ�G��������H�����������ñ�װ�ø�ͭ������GӦ�����������Һ����Ҫ�ɷ���AgNO3��

����Ŀ��(��ǹ�ҵ���Ʊ�Na2S2O3�ķ���֮һ����Ӧԭ��Ϊ��2Na2S+Na2CO3+4SO2=3Na2S2O3+CO2���÷�Ӧ��H��0����ij�о�С����ʵ��������Ʊ�Na2S2O3��5H2O�������¡�
![]()
��1������װ����ͼ��ʾ
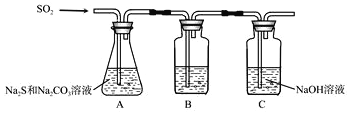
��װ��B�������Ǽ���װ��A��SO2������Ч�ʣ�B���Լ���______������SO2����Ч�ʵ͵�ʵ��������B����Һ_________��
��Ϊ��ʹSO2������������ȫ���ڲ��ı�A����ҺŨ�ȡ�����������£����˼�ʱ���跴Ӧ���⣬���ɲ�ȡ�ĺ�����ʩ��___________________������һ����
��2�����豾ʵ�����õ�Na2CO3������NaCl��NaOH�����ʵ�鷽�����м��顣����ɸ�ʵ�鷽��������֪������ʱCaCO3������Һ��pH��10.2��
��� | ʵ����� | Ԥ������ | ���� |
�� | ȡ������Ʒ���Թ��У�������������ˮ��������ܽ⣬ ______�� | �а�ɫ�������� | ��Ʒ��NaCl |
�� | ��ȡ������Ʒ���ձ��У�������������ˮ����ֽ����ܽ⣬_____�� | �а�ɫ�������ɣ��ϲ���ҺpH>10.2 | ��Ʒ��NaOH |
��3��Na2S2O3��Һ�Ƕ���ʵ���еij����Լ����ⶨ��Ũ�ȵĹ������£�
��һ����ȷ��ȡa g KIO3����ѧʽ����214�����������Һ��
�ڶ������������KI�����H2SO4��Һ���μ�ָʾ����
����������Na2S2O3��Һ�ζ����յ㣬����Na2S2O3��Һ�����ΪV mL����c(Na2S2O3)��___mol��L-1��
��4����(3)��ʵ���У�ijͬѧ��һ���͵ڶ����IJ������ܹ淶������������̫����������õ�Na2S2O3Ũ�ȿ���___________����������Ӱ��������ƫ��������ƫ��������ԭ����_____________��(�����ӷ���ʽ��ʾ)��(��֪��IO3-��5I-+6H+= 3I2��3H2O��4I-��O2+4H+=I2��2H2O��2S2O32-��I2=S4O62-��2I-)