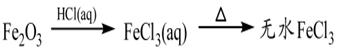��Ŀ����
θ������dz�����θ�����ס���������ѧ�α�����������θ�����ij���θҩ�����������ǵ�˵��ժҪ��
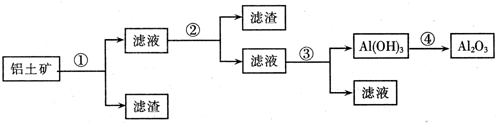
�ף���1����ɫ�ᾧ״��ĩ���ɻ����ֽ⣻��2��������ˮ��ˮ��Һ�������ԣ���3�����ἰ����ҩ�������������̼����4��θ�����߷��ö��θ����������������θ����Σ�ա�
�ң���1����θ�����к����û������־ã���ά��3��4Сʱ����2�����������������������ϣ����б����� �á���3��������ϡ�������������Һ�С�
��1�������Ʋ⣬���к��е���Ҫ��ѧ�ɷ��� ���ѧʽ�������к��е���Ҫ��ѧ�ɷ��� ���ѧʽ����
��2����д�����к��е���Ҫ��ѧ�ɷ�����θ������Ӧ�����ӷ���ʽ�� ��
��3����д�����к��е���Ҫ��ѧ�ɷݷֱ���ϡ���������������Һ��Ӧ�����ӷ���ʽ��
�� ��
�ף���1����ɫ�ᾧ״��ĩ���ɻ����ֽ⣻��2��������ˮ��ˮ��Һ�������ԣ���3�����ἰ����ҩ�������������̼����4��θ�����߷��ö��θ����������������θ����Σ�ա�
�ң���1����θ�����к����û������־ã���ά��3��4Сʱ����2�����������������������ϣ����б����� �á���3��������ϡ�������������Һ�С�
��1�������Ʋ⣬���к��е���Ҫ��ѧ�ɷ��� ���ѧʽ�������к��е���Ҫ��ѧ�ɷ��� ���ѧʽ����
��2����д�����к��е���Ҫ��ѧ�ɷ�����θ������Ӧ�����ӷ���ʽ�� ��
��3����д�����к��е���Ҫ��ѧ�ɷݷֱ���ϡ���������������Һ��Ӧ�����ӷ���ʽ��
�� ��
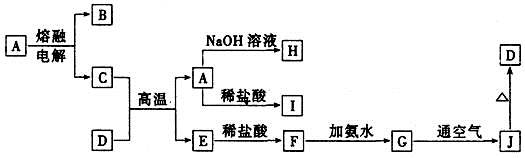
��ÿ��2�֣���10�֣���1��NaHCO3�� Al(OH)3
��2�� HCO3�� �� H�� �� H2O ��CO2��
��3�� Al(OH)3�� 3H�� �� Al3�� �� 3H2O Al(OH)3�� OH�� �� AlO2�� �� 2H2O
��2�� HCO3�� �� H�� �� H2O ��CO2��
��3�� Al(OH)3�� 3H�� �� Al3�� �� 3H2O Al(OH)3�� OH�� �� AlO2�� �� 2H2O
�����������1�����ἰ����ҩ�������������̼��˵������̼������ӻ�̼��������ӣ�ˮ��Һ�������ԣ�˵����ǿ�������Σ�����ʱ����Ϊ��ɫ��˵�����������ӣ���ɫ�ᾧ״��ĩ�����ȿɻ����ֽ⣬̼���Ʋ���ֽ⣬����ֻ����̼�����ƣ�������ϡ�������������Һ�У���������һ�����Լ���������������������ϣ����б������ã�˵����������ˮ�����ɽ�״���ʣ����Ը������������������ʴ�Ϊ��NaHCO3��Al��OH��3��
��2��̼�����ƺ�����ķ�Ӧʵ�ʾ���̼��������Ӻ������ӻ�����ˮ�ķ�Ӧ���ʴ�Ϊ��HCO3-+H+�TH2O+CO2����
��3������Al��OH��3��������������ȿ������ᷴӦҲ������Ӧ��
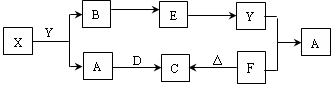
�������ڽ������ʱ�����ȶ������⣬Ȼ����ѧ����֪ʶ�����е�֪ʶ���н����Ҫע�����ʵ�������⡣

��ϰ��ϵ�д�
 ״Ԫ��ȫ��ͻ�Ƶ�����ϵ�д�
״Ԫ��ȫ��ͻ�Ƶ�����ϵ�д�
�����Ŀ