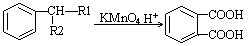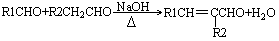��Ŀ����
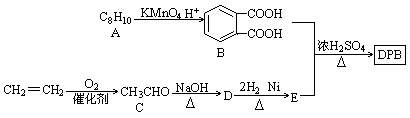
A��һ����Ϣ���ϵ����Ӽ�������ͬ�����£�A������������ͬ�������������88.25������A�����и�Ԫ�����������ֱ�Ϊw��C��=54.4%��w��H��=7.4%��w��O��=18.1%��w��Cl��=20.1%��A�ڲ�ͬ�����¿ɷ�����ͼ��ʾ��һϵ�б仯��

��ش��������⣺
��1��A�ķ���ʽΪ
��2��D���Ӻ��еĹ�������
��3������ת����ϵ�Ļ�ѧ����ʽ�У�����ˮ�ⷴӦ����
��4��д����ѧ����ʽ��
��A��ϡ���Ṳ�ȣ�
��E������G��
��F���������ƴ���Һ���ȣ�
��5����B��Ϊͬ���칹�����������״�������ʹ���

��ش��������⣺
��1��A�ķ���ʽΪ
C8H13O2Cl
C8H13O2Cl
����2��D���Ӻ��еĹ�������
�ǻ�����ԭ��
�ǻ�����ԭ��
����3������ת����ϵ�Ļ�ѧ����ʽ�У�����ˮ�ⷴӦ����
3
3
���������֣�����4��д����ѧ����ʽ��
��A��ϡ���Ṳ�ȣ�
CH2=C��CH3��COOCH2CH��CH3��CH2Cl+H2O
CH2=C��CH3��COOH+ClCH2CH��CH3��CH2OH
| ϡ���� |
| �� |
CH2=C��CH3��COOCH2CH��CH3��CH2Cl+H2O
CH2=C��CH3��COOH+ClCH2CH��CH3��CH2OH
��| ϡ���� |
| �� |
��E������G��
CH3CH��CH2OH��2+2CuO
CH3CH��CHO��2+2Cu+2H2O
| �� |
CH3CH��CH2OH��2+2CuO
CH3CH��CHO��2+2Cu+2H2O
��| �� |
��F���������ƴ���Һ���ȣ�
CH3CH��CH2Cl��COOOH+2NaOH
CH2=C��CH3��COOONa+NaCl+2H2O
| �� |
| �� |
CH3CH��CH2Cl��COOOH+2NaOH
CH2=C��CH3��COOONa+NaCl+2H2O
��| �� |
| �� |
��5����B��Ϊͬ���칹�����������״�������ʹ���
5
5
�֣�����������ͬ�����£�A������������ͬ�������������88.25������Mr��A��=88.25��2=176.5��
��N��C��=
=8��N��H��=
��13��N��O��=
=2��N��Cl��=
��1����A�ķ���ʽΪC8H13O2Cl�����ݿ�ͼ��E��G��G��CH3CH��COOH��2����ط�Ӧ����D������������F����֪��GΪCH3CH��CHO��2��EΪCH3CH��CH2OH��2����DΪCH3CH��CH2Cl��CH2OH���ٸ���D��F��H��B��֪��FΪCH3CH��CH2Cl��COOOH��HΪCH2=C��CH3��COOONa��BΪCH2=C��CH3��COOH����A�Ľṹ��ʽΪCH2=C��CH3��COOCH2CH��CH3��CH2Cl������A�ķ���ʽC8H13O2Cl��B�ķ���ʽΪC4H6O2����̼����������ʽ��C-C-C-C��C-C��C��-C����̼̼˫���Ľṹ�У�C-C-C=C��C-C=C-C��C=C��C��2�������������Ļ������У�HCOOCH2CH=CH2��CH3OOC-CH=CH2��CH3COOCH=CH2��HCOOCH=CH-CH3��CH2=C��CH3��-OOCH����5�֣�
��N��C��=
| 176.5��54.4% |
| 12 |
| 176.5��7.4% |
| 1 |
| 176.5��18.1% |
| 16 |
| 176.5��20.1% |
| 35.5 |
����⣺����ͬ�����£�A������������ͬ�������������88.25������Mr��A��=88.25��2=176.5��
��N��C��=
=8��N��H��=
��13��N��O��=
=2��N��Cl��=
��1����A�ķ���ʽΪC8H13O2Cl�����ݿ�ͼ��E��G��G��CH3CH��COOH��2����ط�Ӧ����D������������F����֪��GΪCH3CH��CHO��2��EΪCH3CH��CH2OH��2����DΪCH3CH��CH2Cl��CH2OH���ٸ���D��F��H��B��֪��FΪCH3CH��CH2Cl��COOOH��HΪCH2=C��CH3��COOONa��BΪCH2=C��CH3��COOH����A�Ľṹ��ʽΪCH2=C��CH3��COOCH2CH��CH3��CH2Cl������A�ķ���ʽC8H13O2Cl��
��1��������������֪��A�ķ���ʽΪC8H13O2Cl��
�ʴ�Ϊ��C8H13O2Cl��
��2��������������֪��DΪCH3CH��CH2Cl��CH2OH�����Ӻ��й�����Ϊ�ǻ�����ԭ�ӣ�
�ʴ�Ϊ���ǻ�����ԭ�ӣ�
��3��A��B��A��C��D��E��ת������ˮ�ⷴӦ������ˮ�ⷴӦ����3����
�ʴ�Ϊ��3��
��4����A��ϡ���Ṳ�ȣ�CH2=C��CH3��COOCH2CH��CH3��CH2Cl+H2O
CH2=C��CH3��COOH+ClCH2CH��CH3��CH2OH��
�ʴ�Ϊ��CH2=C��CH3��COOCH2CH��CH3��CH2Cl+H2O
CH2=C��CH3��COOH+ClCH2CH��CH3��CH2OH��
��E������G��CH3CH��CH2OH��2+2CuO
CH3CH��CHO��2+2Cu+2H2O��
�ʴ�Ϊ��CH3CH��CH2OH��2+2CuO
CH3CH��CHO��2+2Cu+2H2O��
��F���������ƴ���Һ���ȣ�CH3CH��CH2Cl��COOOH+2NaOH
CH2=C��CH3��COOONa+NaCl+2H2O��
�ʴ�Ϊ��CH3CH��CH2Cl��COOOH+2NaOH
CH2=C��CH3��COOONa+NaCl+2H2O��
��5��B�ķ���ʽΪC4H6O2����̼����������ʽ��C-C-C-C��C-C��C��-C����̼̼˫���Ľṹ�У�C-C-C=C��C-C=C-C��C=C��C��2�������������Ļ������У�HCOOCH2CH=CH2��CH3OOC-CH=CH2��CH3COOCH=CH2��HCOOCH=CH-CH3��CH2=C��CH3��-OOCH����5�֣�
�ʴ�Ϊ��5��
��N��C��=
| 176.5��54.4% |
| 12 |
| 176.5��7.4% |
| 1 |
| 176.5��18.1% |
| 16 |
| 176.5��20.1% |
| 35.5 |
��1��������������֪��A�ķ���ʽΪC8H13O2Cl��
�ʴ�Ϊ��C8H13O2Cl��
��2��������������֪��DΪCH3CH��CH2Cl��CH2OH�����Ӻ��й�����Ϊ�ǻ�����ԭ�ӣ�
�ʴ�Ϊ���ǻ�����ԭ�ӣ�
��3��A��B��A��C��D��E��ת������ˮ�ⷴӦ������ˮ�ⷴӦ����3����
�ʴ�Ϊ��3��
��4����A��ϡ���Ṳ�ȣ�CH2=C��CH3��COOCH2CH��CH3��CH2Cl+H2O
| ϡ���� |
| �� |
�ʴ�Ϊ��CH2=C��CH3��COOCH2CH��CH3��CH2Cl+H2O
| ϡ���� |
| �� |
��E������G��CH3CH��CH2OH��2+2CuO
| �� |
�ʴ�Ϊ��CH3CH��CH2OH��2+2CuO
| �� |
��F���������ƴ���Һ���ȣ�CH3CH��CH2Cl��COOOH+2NaOH
| �� |
| �� |
�ʴ�Ϊ��CH3CH��CH2Cl��COOOH+2NaOH
| �� |
| �� |
��5��B�ķ���ʽΪC4H6O2����̼����������ʽ��C-C-C-C��C-C��C��-C����̼̼˫���Ľṹ�У�C-C-C=C��C-C=C-C��C=C��C��2�������������Ļ������У�HCOOCH2CH=CH2��CH3OOC-CH=CH2��CH3COOCH=CH2��HCOOCH=CH-CH3��CH2=C��CH3��-OOCH����5�֣�
�ʴ�Ϊ��5��
���������⿼���л�����ƶ���ϳɣ����չ����ŵ�������ת���ǽ���ؼ�������E�Ķ����������P������������F�����ƶϣ���ѧ�������������нϸߵ�Ҫ���Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ

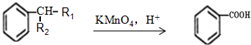



 ��
��