��Ŀ����
��4 mol A�����2 mol B������2 L�������л�ϲ���һ�������·������·�Ӧ2A(g)��B(g)  2C(g)����2 s(��)����C��Ũ��Ϊ0.6 mol/L���������м���˵����
2C(g)����2 s(��)����C��Ũ��Ϊ0.6 mol/L���������м���˵����
��������A��ʾ��Ӧ��ƽ������Ϊ0.3 mol/(L��s)
��������B��ʾ��Ӧ��ƽ������Ϊ0.6 mol/(L��s)
��2 sʱ����A��ת����Ϊ70%
��2 sʱ����B��Ũ��Ϊ0.7 mol/L
������ȷ����(����)
 2C(g)����2 s(��)����C��Ũ��Ϊ0.6 mol/L���������м���˵����
2C(g)����2 s(��)����C��Ũ��Ϊ0.6 mol/L���������м���˵������������A��ʾ��Ӧ��ƽ������Ϊ0.3 mol/(L��s)
��������B��ʾ��Ӧ��ƽ������Ϊ0.6 mol/(L��s)
��2 sʱ����A��ת����Ϊ70%
��2 sʱ����B��Ũ��Ϊ0.7 mol/L
������ȷ����(����)
| A���٢� | B���٢� | C���ڢ� | D���ۢ� |
B
2A(g)��B(g)
 2C(g)
2C(g)4 2 0
1.2 0.6 1.2
2.8 1.4 1.2����λ��Ϊmol��
��Ϲ�ʽ������ô𰸡�����������B��ʾ��Ӧ��ƽ������Ϊ0.15 mol/(L��s)��2 sʱ����A��ת����Ϊ30%

��ϰ��ϵ�д�
�����Ŀ
 2SO3(g) �������й�˵����ȷ���� �� ��
2SO3(g) �������й�˵����ȷ���� �� ��
 CO2+NO��2 minʱ�����������NO�����ʵ���Ϊ0.5 mol ����
CO2+NO��2 minʱ�����������NO�����ʵ���Ϊ0.5 mol ����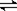 B(g)+D(s)�����ֲ�ͬ�����½��У���Ӧ����Ϊ��ͬ�����ܱ�������B��D��ʼΪ0����Ӧ��A��Ũ�ȣ�mol��L?1���淴Ӧʱ�䣨min���ı仯������±���
B(g)+D(s)�����ֲ�ͬ�����½��У���Ӧ����Ϊ��ͬ�����ܱ�������B��D��ʼΪ0����Ӧ��A��Ũ�ȣ�mol��L?1���淴Ӧʱ�䣨min���ı仯������±���
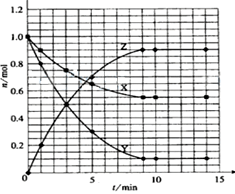
 2Z
2Z 4C2(g) + 2D2 (g) ��5L�ܱ������н��У�����Ӻ�C2�����ʵ���������0.3mol����˷�Ӧ��ƽ������
4C2(g) + 2D2 (g) ��5L�ܱ������н��У�����Ӻ�C2�����ʵ���������0.3mol����˷�Ӧ��ƽ������ x
x A���� B���٢� C���ڢ� D����
A���� B���٢� C���ڢ� D����