��Ŀ����
 ��ѧ��һֱ�����ڡ��˹��̵������·����о���
��ѧ��һֱ�����ڡ��˹��̵������·����о�����1����ͳ���˹��̵����ķ�ӦΪN2��g��+3H2��g��?2NH3��g����һ���¶��£���1molN2��3molH2ͨ�뵽���Ϊ0.5L���ܱ������У��ﵽƽ��״̬ʱH2��ת����Ϊ50%�����¶��¸÷�Ӧ��ƽ�ⳣ��K=
| 4 |
| 27 |
| 4 |
| 27 |
��2�����¡��˹��̵������о����������³�ѹ�����������£�N2�ڴ���������ˮ������Ӧ��2N2��g��+6H2O��l���T4NH3 ��g��+3O2��g����H=Q kJ?mol-1
����֪�÷�Ӧ��ƽ�ⳣ��K���¶ȵĹ�ϵ����ͼ����˷�Ӧ��Q
��
��
0 ���������������=�������ڽ���Ӧ���ɵĻ������ͨ��ˮ�м��ɵð�ˮ����ˮϡ��0.1mol?L-1��ˮ����Һ������ˮ�������Ӷ��������
D
D
������ĸ��ţ���A��
| c(NH3?H2O) |
| c(OH-) |
| c(NH4+)?c(OH-) |
| c(NH3?H2O) |
C��c��H+��?c��OH-�� D��
| c(H+) |
| c(OH-) |
��3���������������������Ҫ��ӦΪ��4NH3��g��+5O2��g���T4NO��g��+6H2O��g����H��0�����ݻ�Ϊ1�����ܱ������з����÷�Ӧ�������ڲ������ʵĺ������±���
| ʱ��/���ʵ��� | n��NH3�� ��mol�� | n��O2 �� ��mol�� | n��NO�� ��mol�� |
| ��ʼ | 1.600 | 3.200 | 0.000 |
| ��2min | a | 2.700 | 0.4000 |
| ��4min | 0.600 | 1.950 | 1.000 |
| ��6min | 0.600 | 1.950 | 1.000 |
0.3
0.3
mol/��L?min�����ڶ���������Ӧ�������жϣ���ȷ����
D
D
������ţ���A�������߷�Ӧ��ϵ���¶ȣ�ƽ��ʱ��ϵ��NH3%����С
B��������Ӧ��ϵ��ѹǿ��ƽ��ʱ��ϵ��NO%������
C�������߷�Ӧ��ϵ���¶ȣ�ƽ��ʱ��ϵ��H2O%������
D�����۸÷�Ӧ�Ƿ��ڴ������ڵ������½��У�ƽ��ʱ��ϵ��O2%���ֲ��䣮
��������1������������Ũ�ȱ仯������������ʽ����ƽ��ʱ����ֵ�ƽ��Ũ�ȣ�����ƽ�ⳣ������ʽ���㣻
��2������ͼ��֪���¶�Խ��ƽ�ⳣ��Խ��˵�������¶�ƽ��������Ӧ�����ƶ����ݴ˽��
����ˮϡ��0.1mol/L��ˮʱ����Һ������ˮ�������ӣ�NH3��H2O?OH-+NH4+�������ƶ���
A��ƽ���������ƶ���n��NH3��H2O�����٣�n��OH-�����ݴ��жϣ�
B������ƽ�ⳣ��ֻ���¶�Ӱ�죻
C��һ���¶��£�ˮ�����ӻ�Ϊ��ֵ��
D��һ���¶��£�ˮ�����ӻ����䣬��ϡ�Ͱ�ˮʱ����Һ����������Ũ�ȼ�С�������ӵ�Ũ�����ӣ��ݴ��жϣ�
��3���ٸ���v=
����v��NO�����ٸ�������֮�ȵ��ڻ�ѧ������֮�ȼ���v��NH3����
��A������ӦΪ���ȷ�Ӧ�������¶ȣ�ƽ�����淴Ӧ�����ƶ������������ʵ�����������������ʵ�����С���ݴ��жϣ�
B������ӦΪ�����������ķ�Ӧ������Ӧ��ϵ��ѹǿ��ƽ�����淴Ӧ�����ƶ���NO�����ʵ������������ڻ�����������ʵ�����������
C������ӦΪ���ȷ�Ӧ�������¶ȣ�ƽ�����淴Ӧ�����ƶ���ˮ�����ʵ������������ڻ�����������ʵ����ļ�������
D��������Ӱ��ƽ���ƶ���
��2������ͼ��֪���¶�Խ��ƽ�ⳣ��Խ��˵�������¶�ƽ��������Ӧ�����ƶ����ݴ˽��
����ˮϡ��0.1mol/L��ˮʱ����Һ������ˮ�������ӣ�NH3��H2O?OH-+NH4+�������ƶ���
A��ƽ���������ƶ���n��NH3��H2O�����٣�n��OH-�����ݴ��жϣ�
B������ƽ�ⳣ��ֻ���¶�Ӱ�죻
C��һ���¶��£�ˮ�����ӻ�Ϊ��ֵ��
D��һ���¶��£�ˮ�����ӻ����䣬��ϡ�Ͱ�ˮʱ����Һ����������Ũ�ȼ�С�������ӵ�Ũ�����ӣ��ݴ��жϣ�
��3���ٸ���v=
| ||
| ��t |
��A������ӦΪ���ȷ�Ӧ�������¶ȣ�ƽ�����淴Ӧ�����ƶ������������ʵ�����������������ʵ�����С���ݴ��жϣ�
B������ӦΪ�����������ķ�Ӧ������Ӧ��ϵ��ѹǿ��ƽ�����淴Ӧ�����ƶ���NO�����ʵ������������ڻ�����������ʵ�����������
C������ӦΪ���ȷ�Ӧ�������¶ȣ�ƽ�����淴Ӧ�����ƶ���ˮ�����ʵ������������ڻ�����������ʵ����ļ�������
D��������Ӱ��ƽ���ƶ���
����⣺��1��������Ũ�ȱ仯��=
=3mol/L����
N2��g��+3H2��g��?2NH3��g��
��ʼ��mol/L����2 6 0
�仯��mol/L����1 3 2
ƽ�⣨mol/L����1 3 2
��ƽ�ⳣ��k=
=
=
��
�ʴ�Ϊ��
��
��2������ͼ��֪���¶�Խ��ƽ�ⳣ��Խ��˵�������¶�ƽ��������Ӧ�����ƶ�������ӦΪ���ȷ�Ӧ����Q��0���ʴ�Ϊ������
����ˮϡ��0.1mol/L��ˮʱ����Һ������ˮ�������ӣ�NH3��H2O?OH-+NH4+�������ƶ���
A��ƽ���������ƶ���n��NH3��H2O�����٣�n��OH-������
ֵ��С����A�����ϣ�
B������ƽ�ⳣ��ֻ���¶�Ӱ�죬
���䣬��B�����ϣ�
C��һ���¶��£�ˮ�����ӻ�Ϊ��ֵ����c��H+��?c��OH-�����䣬��C�����ϣ�
D��һ���¶��£�ˮ�����ӻ����䣬��ϡ�Ͱ�ˮʱ����Һ����������Ũ�ȼ�С�������ӵ�Ũ�����ӣ���
����D���ϣ�
�ʴ�Ϊ��D��
��3����v��NO��=
=0.3mol/��L��min��������֮�ȵ��ڻ�ѧ������֮�ȣ���v��NH3��=v��NO��=0.3mol/��L��min�����ʴ�Ϊ��0.3mol/��L��min����
��A������ӦΪ���ȷ�Ӧ�������¶ȣ�ƽ�����淴Ӧ�����ƶ������������ʵ�����������������ʵ�����С����ƽ��ʱ��ϵ��NH3%������A����
B������ӦΪ�����������ķ�Ӧ������Ӧ��ϵ��ѹǿ��ƽ�����淴Ӧ�����ƶ���NO�����ʵ������������ڻ�����������ʵ�������������ƽ��ʱ��ϵ��NO%����С����B����
C������ӦΪ���ȷ�Ӧ�������¶ȣ�ƽ�����淴Ӧ�����ƶ���ˮ�����ʵ������������ڻ�����������ʵ����ļ���������ƽ��ʱ��ϵ��H2O%����С����C����
D��������Ӱ��ƽ���ƶ���ƽ��ʱ��ϵ��O2%���ֲ��䣬��D��ȷ��
�ʴ�Ϊ��D��
| 3mol��50% |
| 0.5L |
N2��g��+3H2��g��?2NH3��g��
��ʼ��mol/L����2 6 0
�仯��mol/L����1 3 2
ƽ�⣨mol/L����1 3 2
��ƽ�ⳣ��k=
| c2(NH3) |
| c(N2)��c3(H2O) |
| 22 |
| 1��33 |
| 4 |
| 27 |
�ʴ�Ϊ��
| 4 |
| 27 |
��2������ͼ��֪���¶�Խ��ƽ�ⳣ��Խ��˵�������¶�ƽ��������Ӧ�����ƶ�������ӦΪ���ȷ�Ӧ����Q��0���ʴ�Ϊ������
����ˮϡ��0.1mol/L��ˮʱ����Һ������ˮ�������ӣ�NH3��H2O?OH-+NH4+�������ƶ���
A��ƽ���������ƶ���n��NH3��H2O�����٣�n��OH-������
| c(NH3?H2O) |
| c(OH-) |
B������ƽ�ⳣ��ֻ���¶�Ӱ�죬
| c(NH4+)?c(OH-) |
| c(NH3?H2O) |
C��һ���¶��£�ˮ�����ӻ�Ϊ��ֵ����c��H+��?c��OH-�����䣬��C�����ϣ�
D��һ���¶��£�ˮ�����ӻ����䣬��ϡ�Ͱ�ˮʱ����Һ����������Ũ�ȼ�С�������ӵ�Ũ�����ӣ���
| c(H+) |
| c(OH-) |
�ʴ�Ϊ��D��
��3����v��NO��=
| ||
| 4min-2min |
��A������ӦΪ���ȷ�Ӧ�������¶ȣ�ƽ�����淴Ӧ�����ƶ������������ʵ�����������������ʵ�����С����ƽ��ʱ��ϵ��NH3%������A����
B������ӦΪ�����������ķ�Ӧ������Ӧ��ϵ��ѹǿ��ƽ�����淴Ӧ�����ƶ���NO�����ʵ������������ڻ�����������ʵ�������������ƽ��ʱ��ϵ��NO%����С����B����
C������ӦΪ���ȷ�Ӧ�������¶ȣ�ƽ�����淴Ӧ�����ƶ���ˮ�����ʵ������������ڻ�����������ʵ����ļ���������ƽ��ʱ��ϵ��H2O%����С����C����
D��������Ӱ��ƽ���ƶ���ƽ��ʱ��ϵ��O2%���ֲ��䣬��D��ȷ��
�ʴ�Ϊ��D��
���������⿼�黯ѧƽ��ͼ��Ӧ���ʼ��㡢��ѧƽ�ⳣ�����йؼ��㡢��ѧƽ���Ӱ�����صȣ��Ѷ��еȣ���3��ע��ƽ��������Ӧ�����ƶ�����һ��������ĺ�������Ӧ�ﺬ�����ͣ����淴ӦҲ����ˣ����ݸ���ֵı仯�����ܵı仯�������жϣ�

��ϰ��ϵ�д�
 �����ѧСѧ�꼶�νӽݾ��㽭��ѧ������ϵ�д�
�����ѧСѧ�꼶�νӽݾ��㽭��ѧ������ϵ�д�
�����Ŀ

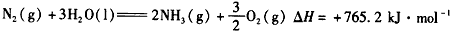
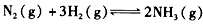 �������ݻ�Ϊ2.0 L���� �������г���0. 60 mol N2(g)��l.60 mol H2(g)����Ӧ��һ�������´ﵽƽ��ʱ��NH3�����ʵ���������NH3�����ʵ����뷴Ӧ��ϵ���ܵ����ʵ���֮�ȣ�Ϊ4/7������
�������ݻ�Ϊ2.0 L���� �������г���0. 60 mol N2(g)��l.60 mol H2(g)����Ӧ��һ�������´ﵽƽ��ʱ��NH3�����ʵ���������NH3�����ʵ����뷴Ӧ��ϵ���ܵ����ʵ���֮�ȣ�Ϊ4/7������ 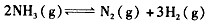 ��ƽ�ⳣ����
��ƽ�ⳣ����