��Ŀ����
2007��10��24�գ��ҹ����������Ƿ��������á��������żס����ػ�������϶�һ�š�̽�����dzɹ�����̫�գ���������㺮�������ĵ�һ�������������żס�������Һ�����ƻ����һ������Ϊ����ȼ�ϣ�������ΪҺ̬��ȼ�ϣ���1������ȼ��ͨ��ָ���£�N2H4��Ϊȼ�ϣ��Զ�������������������������Ϊ���÷��������������������������Ӧ�ͷŵ������������߷�Ӧ���ɵ����ͷ��������壩��
��֪��N2H4��g��+O2��g��=N2��g��+2H2O��g������H=-543kJ?mol-1
 H2��g��+
H2��g��+ F2��g��=HF��g������H=-269kJ?mol-1
F2��g��=HF��g������H=-269kJ?mol-1H2��g��+
 O2��g��=H2O��g������H=-242kJ?mol-1
O2��g��=H2O��g������H=-242kJ?mol-1��д���ºͷ�����Ӧ���Ȼ�ѧ����ʽ��______��
��2������һ���������ɫ��Դ�������ѧ������̫����ֽ�ˮ���Ʊ��⣮��ͼΪ��ֽ�ˮ�����ѭ��ϵͳ����Ӧ����������ĵ�����̫���ܹ����ṩ����Ӧ��ϵ��I2��Fe2+�ȿ�ѭ��ʹ�ã�
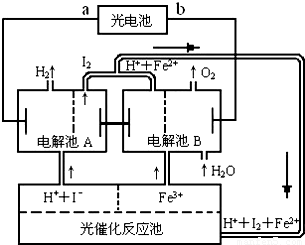
���жϹ��ص�a��Ϊ______����b��Ϊ______����
��д������A�е缫��Ӧʽ��
����______������______��
�۵���B�з�Ӧ�����ӷ���ʽ______ O2��+4H++4Fe2+
���𰸡���������1�����ø�˹������Ѱ����֪�ķ�Ӧ��Ŀ�귴Ӧ�Ĺ�ϵ��Ȼ�����δ֪��Ӧ�ķ�Ӧ�ȣ�����д�Ȼ�ѧ��Ӧ����ʽ��
��2�����õ�����������������Դ�������������õ���ԭ�����������ӵ��ƶ����缫��Ӧʽ�������õ����غ��������йصļ��������
����⣺��1����N2H4��g��+O2��g��=N2��g��+2H2O��g������H=-543kJ?mol-1��
 H2��g��+
H2��g��+ F2��g��=HF��g������H=-269kJ?mol-1��
F2��g��=HF��g������H=-269kJ?mol-1��
H2��g��+ O2��g��=H2O��g������H=-242kJ?mol-1��
O2��g��=H2O��g������H=-242kJ?mol-1��
���ø�˹���ɿ�֪��+��×4-��×2�ɵ÷�ӦN2H4��g��+2F2��g��=N2��g��+4HF��g����
�÷�Ӧ�ġ�H=��-543kJ?mol-1��+4×��-269kJ?mol-1��-2×��-242kJ?mol-1��=-1135kJ?mol-1��
�ʴ�Ϊ��N2H4��g��+2F2��g��=N2��g��+4HF��g����H=-1135kJ?mol-1
��2��������߷ų��������������ڴ˵õ����ӣ������Ϊ��������������a��Ϊԭ��ظ�����b��Ϊ�����������ڴ�ʧȥ���ӣ�
�ʴ�Ϊ���ٸ�����
��2H++2e-��H2����2I-��I2+2e-
��4Fe3++2H2O O2��+4H++4Fe2+
O2��+4H++4Fe2+
��4����ԭ���ԭ������ʧ�����غ���n��H2��=V/Vm=8.96L/22.4molL-1=0.4mol������ �ʴ�Ϊ��ʧȥ������Ϊ2×0.4mol=0.8mol��Fe3+��Fe2+���õ�������Ϊ2×0.4mol
�ʴ�Ϊ��0.8mol
����������Ŀ���ߣ����ͣ��������ݸ�˹�����Լ�������ԭ��Ӧԭ��������ԭ�����Ϳ��Խ�����йص��ӵ�ʧ��ѧ�����ѵ㣮
��2�����õ�����������������Դ�������������õ���ԭ�����������ӵ��ƶ����缫��Ӧʽ�������õ����غ��������йصļ��������
����⣺��1����N2H4��g��+O2��g��=N2��g��+2H2O��g������H=-543kJ?mol-1��
 H2��g��+
H2��g��+ F2��g��=HF��g������H=-269kJ?mol-1��
F2��g��=HF��g������H=-269kJ?mol-1��H2��g��+
 O2��g��=H2O��g������H=-242kJ?mol-1��
O2��g��=H2O��g������H=-242kJ?mol-1�����ø�˹���ɿ�֪��+��×4-��×2�ɵ÷�ӦN2H4��g��+2F2��g��=N2��g��+4HF��g����
�÷�Ӧ�ġ�H=��-543kJ?mol-1��+4×��-269kJ?mol-1��-2×��-242kJ?mol-1��=-1135kJ?mol-1��
�ʴ�Ϊ��N2H4��g��+2F2��g��=N2��g��+4HF��g����H=-1135kJ?mol-1
��2��������߷ų��������������ڴ˵õ����ӣ������Ϊ��������������a��Ϊԭ��ظ�����b��Ϊ�����������ڴ�ʧȥ���ӣ�
�ʴ�Ϊ���ٸ�����
��2H++2e-��H2����2I-��I2+2e-
��4Fe3++2H2O
 O2��+4H++4Fe2+
O2��+4H++4Fe2+ ��4����ԭ���ԭ������ʧ�����غ���n��H2��=V/Vm=8.96L/22.4molL-1=0.4mol������ �ʴ�Ϊ��ʧȥ������Ϊ2×0.4mol=0.8mol��Fe3+��Fe2+���õ�������Ϊ2×0.4mol
�ʴ�Ϊ��0.8mol
����������Ŀ���ߣ����ͣ��������ݸ�˹�����Լ�������ԭ��Ӧԭ��������ԭ�����Ϳ��Խ�����йص��ӵ�ʧ��ѧ�����ѵ㣮

��ϰ��ϵ�д�
�����Ŀ
2007��10��24�գ��ҹ���̽�����ǡ��϶�һ�š�˳�����գ�����������������������ֿ���Ϊ�˾۱���ϵ�
He�������й�
He��˵����ȷ���ǣ�������
3 2 |
3 2 |
| A��������Ϊ2 |
| B�����������Ϊ3 |
| C��������Ϊ3 |
| D��������Ϊ2 |