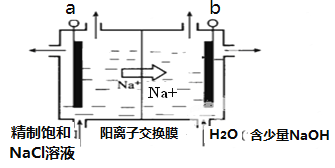
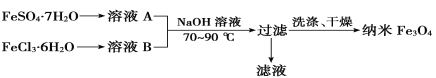
��Ŀ����
����Ŀ����������Ԫ��(����Ԫ����ȥ)�����ڱ��е�λ������:

(1) A��Ԫ�ط�����________��
(2) D�����ڱ��е�λ����_________________________________��
(3) E��G��T��ԭ�Ӱ뾶�ɴ�С��˳����_________(��Ԫ�ط���)��
(4)��Ԫ��(34Se)��Dͬ���壬��ǽ����Ա�D_________(����ǿ����������)����ԭ�ӽṹ�ǶȽ�����ԭ��:___________________________________________________________________��
(5) RԪ�ص�ԭ�ӽṹʾ��ͼΪ_________________��
(6) E��G��J����Ԫ������������Ӧˮ��������֮���ܷ�Ӧ�����ӷ���ʽ�ֱ�Ϊ:H+ +OH-��H2O��_________________________________��______________________��
(7) E��D��Ԫ�����γ�ԭ�Ӹ�����2:1��1:1�����ֻ����2:1 �ͻ�����ĵ���ʽΪ______________________________��1:1�ͻ�����Ļ�ѧʽ�Լ�������ѧ��������_____________________________��
(8)A��M�γɵķ��ӿ�����______________(����ĸ���)��

���𰸡�H ��2���ڵ���A�� K��Na��Al �� Se��Oͬ���壬���Ӳ���Se��O��ԭ�Ӱ뾶Se��O���õ�������Se��O���ǽ�����Se��O  Al(OH)3+3H+��A13++3H2O Al(OH)3+OH����AlO2-+2H2O
Al(OH)3+3H+��A13++3H2O Al(OH)3+OH����AlO2-+2H2O ![]() Na2O2 ���Ӽ����Ǽ��Լ� ��
Na2O2 ���Ӽ����Ǽ��Լ� ��
��������
��������Ԫ�������ڱ��е����λ���жϳ�Ԫ�����ƣ�Ȼ����Ԫ�������ɡ����ʵ����ʷ������
����Ԫ�������ڱ��е����λ�ÿ�֪A��H��B��N��D��O��E��Na��G��Al��J��Cl��M��C��R��S��T��K����
(1)A��Ԫ�ط�����H��
(2)D��O�������ڱ��е�λ���ǵ�2���ڵڢ�A�塣
(3)ͬ������������ԭ�Ӱ뾶��С��ͬ������ϵ���ԭ�Ӱ뾶��������E��G��T��ԭ�Ӱ뾶�ɴ�С��˳����K��Na��Al��
(4)ͬ������ϵ��·ǽ�������������Ԫ��(34Se)��Dͬ���壬����ǽ����Ա�D������������Se��Oͬ���壬���Ӳ���Se��O��ԭ�Ӱ뾶Se��O���õ�������Se��O���ǽ�����Se��O��
(5)RԪ����S����ԭ�ӽṹʾ��ͼΪ ��
��
(6)E��G��J����Ԫ������������Ӧˮ����ֱ����������ơ��������������ᣬ����֮���ܷ�Ӧ�����ӷ���ʽ�ֱ�ΪH++OH-��H2O��Al(OH)3+3H+��A13++3H2O��Al(OH)3+OH����AlO2-+2H2O��
(7)E��D��Ԫ�����γ�ԭ�Ӹ�����2:1��1:1�����ֻ�����ֱ��������ƺ������ơ�2:1�ͻ������������ƣ�����ʽΪ![]() ��1:1�ͻ�����Ļ�ѧʽΪNa2O2��������ѧ�����������Ӽ����Ǽ��Լ���
��1:1�ͻ�����Ļ�ѧʽΪNa2O2��������ѧ�����������Ӽ����Ǽ��Լ���
(8)A��M�γɵķ��ӿ����Ǽ��飬����ʽΪCH4����ѡ�ۡ�

 ���Ͱ�ͨ�������Сѧ��ʱͬ�����ϵ�д�
���Ͱ�ͨ�������Сѧ��ʱͬ�����ϵ�д� ���Ͱ�ͨ������ϵ�д�
���Ͱ�ͨ������ϵ�д�����Ŀ��A��B��C��D��E��F��G��Ϊ������Ԫ�أ�ԭ���������ε�����AԪ��ԭ�Ӻ��������ӣ�BԪ��ԭ�������������Ǵ�����������2����D�ǵؿ��к�������Ԫ�أ�E�Ļ�������ɫ��Ӧ�ǻ�ɫ��F��Gλ�����ڣ�G��ͬ����Ԫ����ԭ�Ӱ뾶��С��Ԫ�ء�
���û�ѧ����ش�
��1��D�ļ������ӵĽṹʾ��ͼ��___________��
��2���õ���ʽ��ʾE2F���γɹ���____________��
��3��E��F��G����Ԫ�����γɵļ����ӣ��뾶�ɴ�С��˳����_________>_________>_________��
��4������ʵ�������Ӧ��ʵ�������У�����ȷ����_________������ĸ����
ѡ�� | ʵ����� | ʵ������ |
a | ��E����Ͷ�뵽CuSO4��Һ�� | ���ɴ�����ɫ���� |
b | ��AlCl3��Һ��ͨ�����C����̬�⻯�� | �����ɰ�ɫ������Ȼ������ܽ� |
c | ��G�ĵ���ͨ�뵽NaBr��Һ�г�ַ�Ӧ�������Ȼ�̼�������� | �²���Һ��Ϊ��ɫ |
d | ��B�����������ͨ�뵽Na2SiO3��Һ�� | ���ɰ�ɫ���� |
��5��д��A��B�γɵ�10���ӷ��ӵĻ�ѧʽ_________����������G�ĵ����ڹ����·�Ӧ��һ��ʱ�������װ��ʾ��ͼ������ȷ��ӳʵ���������_________������ĸ����

��6����Fe��Cu�Ļ�����У�����һ������C������������Ӧ��ˮ�����ϡ��Һ����ַ�Ӧ��ʣ�����m1 g���������м���һ������ϡ���ᣬ��ַ�Ӧ��ʣ�����m2 g������˵����ȷ����_________������ĸ����
a m1����m2 b m1����m2
c ʣ����Һ��һ����Fe3+ d ʣ�������һ����Cu
����Ŀ�����ܱ������з������з�Ӧ��I2(g)��H2(g) ![]() 2HI(g)������Ӧ���ȣ�����ʼʱ��n(H2)��a mol��n(I2)��b mol��ֻ�ı�������г��������������������䣬�Խ���ѧ��Ӧ���ʵĸı䣨����������С�����䡱��������Ӧ�ı���
2HI(g)������Ӧ���ȣ�����ʼʱ��n(H2)��a mol��n(I2)��b mol��ֻ�ı�������г��������������������䣬�Խ���ѧ��Ӧ���ʵĸı䣨����������С�����䡱��������Ӧ�ı���
��� | ��Ӧ���� | ��Ӧ���� |
��1�� | �����¶� | ____________ |
��2�� | ������� | _____________ |
��3�� | �ٳ���a mol H2 | _____________ |
��4�� | �������ݻ�����Ϊԭ��2�� | ___________ |
��5�� | ͨ��b mol Ne(g) | __________ |