��Ŀ����
�ҹ�ũҵ�������������ɵ���ʧÿ��ߴ�15�ڶ�Ԫ��Ϊ����Ч�������꣬Ŀǰ����Ժ�����ˡ�����������Ͷ���������Ⱦ���������ַ������ȷ��森
��1��������ˮ��Ʒ1�ݣ�ÿ��һ��ʱ��ⶨ����ˮ��Ʒ��pH�����������£�
�������ݣ��ش��������⣺
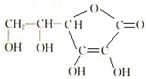
����ˮ��Ʒ��PH�仯��ԭ���û�ѧ����ʽ��ʾ���� ��
���������ȡ����������ˮ������ˮ��ͣ�PH���� ���������С���������䡱��
��2������Ϊ�������������;���ɲ��õĴ�ʩ�� ��
������ú��ȼ�� �ڰѹ����̴���� ��ȼ������ �������ữ�������м�ʯ�� �ݿ�������Դ
A���٢ڢ�B���ڢۢܢ�C���٢ڢ�D���٢ۢ�
��3����Ӣ��������һ���о���������������̴ѿ�����Ч�ؽ�������Χ�ر���SO2Ũ�ȣ���20���͵�60��70�����10��䣬�ɷ��糧�ŷų���SO2������35%�������ڽ������̴ѣ����ʹ��������SO2Ũ�Ƚ�����30%֮�࣮�����ȫ�������ĽǶȣ��������ַ����Ƿ��ȡ������������ ��
��1��������ˮ��Ʒ1�ݣ�ÿ��һ��ʱ��ⶨ����ˮ��Ʒ��pH�����������£�
| ����ʱ��/h | 0 | 1 | 2 | 3 | 4 |
| ��ˮ��pH | 4.73 | 4.62 | 4.56 | 4.55 | 4.55 |
����ˮ��Ʒ��PH�仯��ԭ���û�ѧ����ʽ��ʾ����
���������ȡ����������ˮ������ˮ��ͣ�PH����
��2������Ϊ�������������;���ɲ��õĴ�ʩ��
������ú��ȼ�� �ڰѹ����̴���� ��ȼ������ �������ữ�������м�ʯ�� �ݿ�������Դ
A���٢ڢ�B���ڢۢܢ�C���٢ڢ�D���٢ۢ�
��3����Ӣ��������һ���о���������������̴ѿ�����Ч�ؽ�������Χ�ر���SO2Ũ�ȣ���20���͵�60��70�����10��䣬�ɷ��糧�ŷų���SO2������35%�������ڽ������̴ѣ����ʹ��������SO2Ũ�Ƚ�����30%֮�࣮�����ȫ�������ĽǶȣ��������ַ����Ƿ��ȡ������������
��������1���������ŷų�����SO2����������ˮ������H2SO3��H2SO3����ˮ���½����������е��������������������H2SO4��2H2SO3+O2=2H2SO4���Ӷ�ʹ��ˮ��������ǿ��
�ڸ�������������H2SO3������������ᣬ��Һ��������ǿ��pH��С��
��2������ú��ȼ�ϡ�ȼ���������µ���Դ�ȴ�ʩ���Լ��ٶ�������������ŷţ��Ӷ�����������γɣ��ѹ����̴���ߡ������ữ�������м�ʯ�ҵȴ�ʩ������Ч�ط�ֹ������γɣ�
��3������������ŷ��������䣬����Ⱦ��Χ���ݴ˷������
�ڸ�������������H2SO3������������ᣬ��Һ��������ǿ��pH��С��
��2������ú��ȼ�ϡ�ȼ���������µ���Դ�ȴ�ʩ���Լ��ٶ�������������ŷţ��Ӷ�����������γɣ��ѹ����̴���ߡ������ữ�������м�ʯ�ҵȴ�ʩ������Ч�ط�ֹ������γɣ�
��3������������ŷ��������䣬����Ⱦ��Χ���ݴ˷������
����⣺��1���ٿ����д���CO2����ˮ����̼��ʹ��ˮ�����ԣ�������ˮ��pHֵԼ����5.6����ˮ��Ʒ��PH��С��ԭ���������ŷų�����SO2��������ˮ��SO2+H2O?H2SO3����H2SO3��H2SO3����ˮ���½����������е��������������������H2SO4��.2H2SO3+O2=2H2SO4���Ӷ�ʹ��ˮ��������ǿ���÷�Ӧ����Ҳ���û�2SO2+2H2O+O2=2H2SO4��ʾ��
�ʴ�Ϊ��SO2+H2O?H2SO3��2H2SO3+O2=2H2SO4��2SO2+2H2O+O2=2H2SO4��
������ˮͨ��������������������������H2SO3������������ᣬ���߶���ǿ�ᣬ����������ǿ��pH��С��
�ʴ�Ϊ��С��
��2���ѹ����̴���ߡ������ữ�������м�ʯ�ҵȴ�ʩ���ܴ�Դͷ��Ч�ط�ֹ������γɣ�����ú��ȼ�ϡ�ȼ���������µ���Դ�ȴ�ʩ���Լ��ٶ�������������ŷţ��Ӷ�����������γɣ�
��ѡ��D��
��3��������Ͳ�ĸ߶���Ȼ����ʹ��Χ�ر���SO2�ĺ������٣����Ƕ���������ŷ��������䣬����Ⱦ��Χ�����ˣ�
�ʴ�Ϊ������ȡ������������ŷ��������䣬����Ⱦ��Χ����
�ʴ�Ϊ��SO2+H2O?H2SO3��2H2SO3+O2=2H2SO4��2SO2+2H2O+O2=2H2SO4��
������ˮͨ��������������������������H2SO3������������ᣬ���߶���ǿ�ᣬ����������ǿ��pH��С��
�ʴ�Ϊ��С��
��2���ѹ����̴���ߡ������ữ�������м�ʯ�ҵȴ�ʩ���ܴ�Դͷ��Ч�ط�ֹ������γɣ�����ú��ȼ�ϡ�ȼ���������µ���Դ�ȴ�ʩ���Լ��ٶ�������������ŷţ��Ӷ�����������γɣ�
��ѡ��D��
��3��������Ͳ�ĸ߶���Ȼ����ʹ��Χ�ر���SO2�ĺ������٣����Ƕ���������ŷ��������䣬����Ⱦ��Χ�����ˣ�
�ʴ�Ϊ������ȡ������������ŷ��������䣬����Ⱦ��Χ����
������������Ҫ����������γɣ����ն�������������ǽ��Ĺؼ�����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ

 ��1���ҹ�ũҵ�������������ɵ���ʧÿ��ߴ�15�ڶ�Ԫ��������ָpHС��
��1���ҹ�ũҵ�������������ɵ���ʧÿ��ߴ�15�ڶ�Ԫ��������ָpHС��