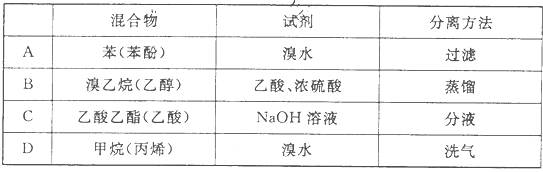
��Ŀ����
��16�֣���ˮż����Ӧ��һ�����͵�ֱ���������Ӧ�����磺
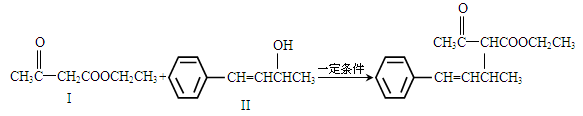
��1��������I�ķ���ʽΪ_____��1mol��������ȫȼ��������Ҫ����_____molO2��
��2��������II��ʹ____��Һ����дһ�֣���ɫ��������III������ʽΪC10H11C1������NaOHˮ��Һ�������ɻ�����II����Ӧ�Ļ�ѧ����ʽΪ______��
��3��������III��NaOH�Ҵ���Һ�������ɻ�����IV��IV�ĺ˴Ź������׳��������������壬�����֮��ΪΪ1��1��1��2��IV�Ľṹ��ʽΪ_______��
��4����CH3COOCH2CH3�ɺϳɻ�����I��������V��CH3COOCH2CH3��һ����֧��ͬ���칹�壬̼�����˳ʶԳƽṹ������Cu���������O2��Ӧ�����ܷ���������Ӧ�Ļ�����VI��V�Ľṹ��ʽΪ______��VI�Ľṹ��ʽΪ______��
��5��һ�������£�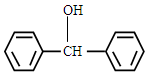 ��
�� Ҳ���Է������Ʒ�Ӧ�ٵķ�Ӧ���л�����Ľṹ��ʽΪ_____��
Ҳ���Է������Ʒ�Ӧ�ٵķ�Ӧ���л�����Ľṹ��ʽΪ_____��

��1��������I�ķ���ʽΪ_____��1mol��������ȫȼ��������Ҫ����_____molO2��
��2��������II��ʹ____��Һ����дһ�֣���ɫ��������III������ʽΪC10H11C1������NaOHˮ��Һ�������ɻ�����II����Ӧ�Ļ�ѧ����ʽΪ______��
��3��������III��NaOH�Ҵ���Һ�������ɻ�����IV��IV�ĺ˴Ź������׳��������������壬�����֮��ΪΪ1��1��1��2��IV�Ľṹ��ʽΪ_______��
��4����CH3COOCH2CH3�ɺϳɻ�����I��������V��CH3COOCH2CH3��һ����֧��ͬ���칹�壬̼�����˳ʶԳƽṹ������Cu���������O2��Ӧ�����ܷ���������Ӧ�Ļ�����VI��V�Ľṹ��ʽΪ______��VI�Ľṹ��ʽΪ______��
��5��һ�������£�
 ��
�� Ҳ���Է������Ʒ�Ӧ�ٵķ�Ӧ���л�����Ľṹ��ʽΪ_____��
Ҳ���Է������Ʒ�Ӧ�ٵķ�Ӧ���л�����Ľṹ��ʽΪ_____����1��C6H10O3��2�֣� 7��2�֣�
��2��������Ȼ�̼�������Ը�����أ����������֣�2�֣�
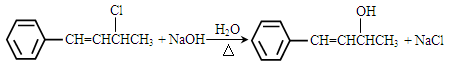
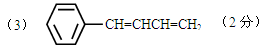
��4��HOCH2CH=CHCH2OH��2�֣� ��2�֣���������д��Ҳ���֣�
��5��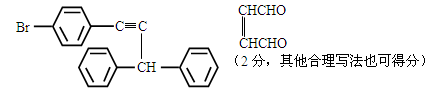
��2��������Ȼ�̼�������Ը�����أ����������֣�2�֣�

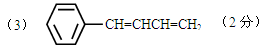
��4��HOCH2CH=CHCH2OH��2�֣� ��2�֣���������д��Ҳ���֣�
��5��

��1����Ӧ���л�����I�Ľṹ��ʽΪCH3COCH2COOCH2CH3����һ����������̼���⡢��ԭ�ӵ���Ŀ���ɵ������ʽΪC6H10O3���������ĺ���������ȼ��ͨʽ�ɵã�C6H10O3+7O2 6CO2+5H2O������������I�����ʵ���֮�ȵ���ϵ��֮�ȣ���1molI��ȫȼ��������Ҫ����7mol O2����2����Ӧ���л�����II�Ĺ�������̼̼˫�����ǻ���ǰ��������Է����ӳɷ�Ӧ��ǰ�ߺͺ��߶��ܱ����Ը��������Һ�������ɴ��ƶ�II����ʹ������Ȼ�̼��Һ����ˮ�����Ը��������Һ��ɫ��II�Ľṹ��ʽΪC6H5CH=CHCHOHCH3�������ʽΪC10H12O��III����±�����������ʽΪC10H11Cl��III��II��1��Cl��1��OH�� III�� NaOHˮ��Һ��������II��˵��III�Ľṹ��ʽΪC6H5CH=CHCHClCH3������±���������ʿ���д����ƽ��ˮ�ⷴӦ�Ļ�ѧ����ʽ����3������±������NaOH�Ҵ���Һ���ȷ�����ȥ��Ӧ��ԭ����III��NaOH�Ҵ���Һ�������ɵ��л����������C6H5CH=C=CHCH3��C6H5CH=CHCH=CH2��ǰ�ߵĺ˴Ź������׳��������������壬�����֮��Ϊ1��1��3����˲��������⣬����IV�Ľṹ��ʽΪC6H5CH=CHCH=CH2����4�������Ǽ�����CH2OH��HOCH2������Cu���������O2��Ӧ�����ܷ���������Ӧ��ȩ������CHO��OHC�������������֪V��̼�����˸���1���Ǽ�����CH2OH��HOCH2������CH3COOCH2CH3�ķ���ʽΪC4H8O2����ȥ2��C��6��H��2��O֮��ɵ�2��C��2��H����V��̼���м�ʣ�����Ϊ��CH=CH������V�Ľṹ��ʽΪHOCH2CH=CHCH2OH���Դ�Ϊͻ�ƿڣ������ƶ�VI�Ľṹ��ʽΪOHCCH=CHCHO����5���ȽϷ�Ӧ���з�Ӧ��I��II��������Ľṹ��ʽ���ҳ�������ͬ�Ļ��źͲ���ͬ�Ļ��ţ�Ȼ���ƶϸ÷�Ӧʵ����II�������ǻ�����̼�����ѣ�I����������ͪ������̼�ϵ�̼������ѣ��ǻ�����������������ˮ�����ಿ�ֽ�������л�����ɴ���ȿɵ����Ʒ�Ӧ�ٵ��л�����Ľṹ��ʽ��
6CO2+5H2O������������I�����ʵ���֮�ȵ���ϵ��֮�ȣ���1molI��ȫȼ��������Ҫ����7mol O2����2����Ӧ���л�����II�Ĺ�������̼̼˫�����ǻ���ǰ��������Է����ӳɷ�Ӧ��ǰ�ߺͺ��߶��ܱ����Ը��������Һ�������ɴ��ƶ�II����ʹ������Ȼ�̼��Һ����ˮ�����Ը��������Һ��ɫ��II�Ľṹ��ʽΪC6H5CH=CHCHOHCH3�������ʽΪC10H12O��III����±�����������ʽΪC10H11Cl��III��II��1��Cl��1��OH�� III�� NaOHˮ��Һ��������II��˵��III�Ľṹ��ʽΪC6H5CH=CHCHClCH3������±���������ʿ���д����ƽ��ˮ�ⷴӦ�Ļ�ѧ����ʽ����3������±������NaOH�Ҵ���Һ���ȷ�����ȥ��Ӧ��ԭ����III��NaOH�Ҵ���Һ�������ɵ��л����������C6H5CH=C=CHCH3��C6H5CH=CHCH=CH2��ǰ�ߵĺ˴Ź������׳��������������壬�����֮��Ϊ1��1��3����˲��������⣬����IV�Ľṹ��ʽΪC6H5CH=CHCH=CH2����4�������Ǽ�����CH2OH��HOCH2������Cu���������O2��Ӧ�����ܷ���������Ӧ��ȩ������CHO��OHC�������������֪V��̼�����˸���1���Ǽ�����CH2OH��HOCH2������CH3COOCH2CH3�ķ���ʽΪC4H8O2����ȥ2��C��6��H��2��O֮��ɵ�2��C��2��H����V��̼���м�ʣ�����Ϊ��CH=CH������V�Ľṹ��ʽΪHOCH2CH=CHCH2OH���Դ�Ϊͻ�ƿڣ������ƶ�VI�Ľṹ��ʽΪOHCCH=CHCHO����5���ȽϷ�Ӧ���з�Ӧ��I��II��������Ľṹ��ʽ���ҳ�������ͬ�Ļ��źͲ���ͬ�Ļ��ţ�Ȼ���ƶϸ÷�Ӧʵ����II�������ǻ�����̼�����ѣ�I����������ͪ������̼�ϵ�̼������ѣ��ǻ�����������������ˮ�����ಿ�ֽ�������л�����ɴ���ȿɵ����Ʒ�Ӧ�ٵ��л�����Ľṹ��ʽ��
���鿼����ϩ����Ȳ����±����������ȩ�����ȳ����л���Ľṹ�ͳɼ��������˽⣻���鿼���������л�����ʹ��������ɺ������Լ��������ϵ���˽⣻���鿼������Ҫ�л���Ӧ������ȡ����Ӧ���ӳɷ�Ӧ����ȥ��Ӧ��������Ӧ���˽⣻���鿼���Ժ˴Ź������������ͷ����֮�ȵ��˽⣻���鿼�����л�������ͬ���칹������˽⣻���鿼���ķ����������������������ۺ�Ӧ����Ϣ�Լ���ϢǨ��������
 6CO2+5H2O������������I�����ʵ���֮�ȵ���ϵ��֮�ȣ���1molI��ȫȼ��������Ҫ����7mol O2����2����Ӧ���л�����II�Ĺ�������̼̼˫�����ǻ���ǰ��������Է����ӳɷ�Ӧ��ǰ�ߺͺ��߶��ܱ����Ը��������Һ�������ɴ��ƶ�II����ʹ������Ȼ�̼��Һ����ˮ�����Ը��������Һ��ɫ��II�Ľṹ��ʽΪC6H5CH=CHCHOHCH3�������ʽΪC10H12O��III����±�����������ʽΪC10H11Cl��III��II��1��Cl��1��OH�� III�� NaOHˮ��Һ��������II��˵��III�Ľṹ��ʽΪC6H5CH=CHCHClCH3������±���������ʿ���д����ƽ��ˮ�ⷴӦ�Ļ�ѧ����ʽ����3������±������NaOH�Ҵ���Һ���ȷ�����ȥ��Ӧ��ԭ����III��NaOH�Ҵ���Һ�������ɵ��л����������C6H5CH=C=CHCH3��C6H5CH=CHCH=CH2��ǰ�ߵĺ˴Ź������׳��������������壬�����֮��Ϊ1��1��3����˲��������⣬����IV�Ľṹ��ʽΪC6H5CH=CHCH=CH2����4�������Ǽ�����CH2OH��HOCH2������Cu���������O2��Ӧ�����ܷ���������Ӧ��ȩ������CHO��OHC�������������֪V��̼�����˸���1���Ǽ�����CH2OH��HOCH2������CH3COOCH2CH3�ķ���ʽΪC4H8O2����ȥ2��C��6��H��2��O֮��ɵ�2��C��2��H����V��̼���м�ʣ�����Ϊ��CH=CH������V�Ľṹ��ʽΪHOCH2CH=CHCH2OH���Դ�Ϊͻ�ƿڣ������ƶ�VI�Ľṹ��ʽΪOHCCH=CHCHO����5���ȽϷ�Ӧ���з�Ӧ��I��II��������Ľṹ��ʽ���ҳ�������ͬ�Ļ��źͲ���ͬ�Ļ��ţ�Ȼ���ƶϸ÷�Ӧʵ����II�������ǻ�����̼�����ѣ�I����������ͪ������̼�ϵ�̼������ѣ��ǻ�����������������ˮ�����ಿ�ֽ�������л�����ɴ���ȿɵ����Ʒ�Ӧ�ٵ��л�����Ľṹ��ʽ��
6CO2+5H2O������������I�����ʵ���֮�ȵ���ϵ��֮�ȣ���1molI��ȫȼ��������Ҫ����7mol O2����2����Ӧ���л�����II�Ĺ�������̼̼˫�����ǻ���ǰ��������Է����ӳɷ�Ӧ��ǰ�ߺͺ��߶��ܱ����Ը��������Һ�������ɴ��ƶ�II����ʹ������Ȼ�̼��Һ����ˮ�����Ը��������Һ��ɫ��II�Ľṹ��ʽΪC6H5CH=CHCHOHCH3�������ʽΪC10H12O��III����±�����������ʽΪC10H11Cl��III��II��1��Cl��1��OH�� III�� NaOHˮ��Һ��������II��˵��III�Ľṹ��ʽΪC6H5CH=CHCHClCH3������±���������ʿ���д����ƽ��ˮ�ⷴӦ�Ļ�ѧ����ʽ����3������±������NaOH�Ҵ���Һ���ȷ�����ȥ��Ӧ��ԭ����III��NaOH�Ҵ���Һ�������ɵ��л����������C6H5CH=C=CHCH3��C6H5CH=CHCH=CH2��ǰ�ߵĺ˴Ź������׳��������������壬�����֮��Ϊ1��1��3����˲��������⣬����IV�Ľṹ��ʽΪC6H5CH=CHCH=CH2����4�������Ǽ�����CH2OH��HOCH2������Cu���������O2��Ӧ�����ܷ���������Ӧ��ȩ������CHO��OHC�������������֪V��̼�����˸���1���Ǽ�����CH2OH��HOCH2������CH3COOCH2CH3�ķ���ʽΪC4H8O2����ȥ2��C��6��H��2��O֮��ɵ�2��C��2��H����V��̼���м�ʣ�����Ϊ��CH=CH������V�Ľṹ��ʽΪHOCH2CH=CHCH2OH���Դ�Ϊͻ�ƿڣ������ƶ�VI�Ľṹ��ʽΪOHCCH=CHCHO����5���ȽϷ�Ӧ���з�Ӧ��I��II��������Ľṹ��ʽ���ҳ�������ͬ�Ļ��źͲ���ͬ�Ļ��ţ�Ȼ���ƶϸ÷�Ӧʵ����II�������ǻ�����̼�����ѣ�I����������ͪ������̼�ϵ�̼������ѣ��ǻ�����������������ˮ�����ಿ�ֽ�������л�����ɴ���ȿɵ����Ʒ�Ӧ�ٵ��л�����Ľṹ��ʽ�����鿼����ϩ����Ȳ����±����������ȩ�����ȳ����л���Ľṹ�ͳɼ��������˽⣻���鿼���������л�����ʹ��������ɺ������Լ��������ϵ���˽⣻���鿼������Ҫ�л���Ӧ������ȡ����Ӧ���ӳɷ�Ӧ����ȥ��Ӧ��������Ӧ���˽⣻���鿼���Ժ˴Ź������������ͷ����֮�ȵ��˽⣻���鿼�����л�������ͬ���칹������˽⣻���鿼���ķ����������������������ۺ�Ӧ����Ϣ�Լ���ϢǨ��������

��ϰ��ϵ�д�
�����Ŀ