��Ŀ����
���й��ڷ�Ӧ�ȵ���������ȷ����
A. ��H��(aq)��OH��(aq)===H2O��l����H����57.3 kJ��mol��1��֪����1 mol CH3COOH����Һ�뺬1 mol NaOH����Һ��ϣ��ų�����Ϊ57.3 kJ
B. ��C(ʯī��s)===C(���ʯ��s)����H��1.9 kJ��mol��1����֪��ʯī�Ƚ��ʯ���ȶ�
C. 500�桢30 MPa�£���0.5 mol N2��1.5 mol H2�����ܱ������г�ַ�Ӧ����NH3(g)������19.3 kJ�����Ȼ�ѧ����ʽΪN2(g)��3H2(g) 2NH3(g) ��H����38.6 kJ��mol��1
2NH3(g) ��H����38.6 kJ��mol��1
D. ����ı�ȼ���ȣ���H��Ϊ��890.3 kJ��mol��1�������ȼ�յ��Ȼ�ѧ����ʽ�ɱ�ʾΪCH4(g)��2O2(g)===CO2(g)��2H2O(g)����H����890.3 kJ��mol��1
 �������Ͽ�ʱͬ��ѵ��ϵ�д�
�������Ͽ�ʱͬ��ѵ��ϵ�д� �������ͬ����ϰϵ�д�
�������ͬ����ϰϵ�д��������£���һԪ��HA����Һ��KOH��Һ�������ϣ����Ի�Ϻ���Һ������仯����ʵ���������±���
ʵ����� | ��ʼŨ�ȣ���mol��L��1�� | ��Ӧ����Һ��pH | |
c��HA�� | c��KOH�� | ||
�� | 0.1 | 0.1 | 9 |
�� | x | 0.2 | 7 |
��ش�
��1��HA��Һ��KOH��Һ��Ӧ�����ӷ���ʽΪ________��
��2��ʵ��ٷ�Ӧ�����Һ����ˮ�������c��OH������________mol��L��1��x________0.2mol��L��1���������������������
��3�����й���ʵ��ڷ�Ӧ�����Һ˵������ȷ����________������ĸ����
a����Һ��ֻ����������ƽ��
b����Һ�У�c��A������c��HA����0.1mol��L��1
c����Һ�У�c��K������c��A������c��OH������c��H����
����֪2H2��g����O2��g����2H2O��1�� ��H����572kJ��mol��1��ij����ȼ�ϵ�������ɶ��ʯī��Ϊ�缫��KOH��ҺΪ�������Һ��
��4��д���õ�ع���ʱ�����ĵ缫��Ӧʽ________��
��5����������ȼ�ϵ��ÿ�ͷ�228.8kJ����ʱ��������1molҺ̬ˮ����õ�ص�����ת����Ϊ________��

 Si3N4��12HCl���йظ÷�Ӧ˵����ȷ����
Si3N4��12HCl���йظ÷�Ӧ˵����ȷ����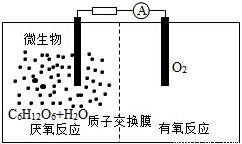
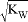 mol?L-1
mol?L-1