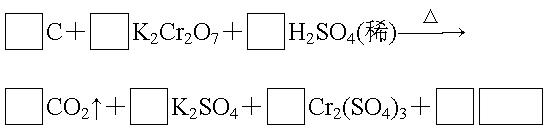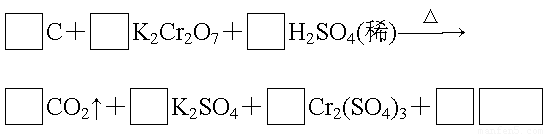��Ŀ����
���������ҹ���̼���ܵ��о�Ҳ���˽ϴ�ͻ�ƣ����õ绡�ϳɷ�����̼���ܣ��������д�����̼���������������������ᴿ��Щ��������ѧ����ʽΪ��

�Իش��������⣺
��1����ɲ���ƽ������Ӧ�Ļ�ѧ����ʽ.
��2���˷�Ӧ��������Ϊ������������������������Ԫ��Ϊ����������������.
��3��H2SO4��������Ӧ�б��ֳ�����������������������ĸ��.
A�����ԡ�����B�������� C����ˮ�� D����ˮ��
��4��������Ӧ������11 g CO2����ת�Ƶ��ӵ���ĿΪ��������������������.
����2009��3�µ���4����Ѯ��ī���硢�����ȶ��������������H1N1�����У����H1N1�������У����飬���Ҳ���������ҹ�����.����ר�ұ�ʾ�������������������������ɷ�����H1N1����.
��1������������Ŀǰ�����Ϲ��ϵĵ��Ĵ���Ч�����Ĺ�����������������KClO3��Һ��H2SO4��������Na2SO3��Һ��Ӧ�Ƶ�.��д���÷�Ӧ�����ӷ���ʽ��
��
��2����̼������һ���ж���;��������ϵ��̬Ư������ѧʽ�ɱ�ʾΪNa2CO3��3H2O2��������Na2CO3��H2O2��˫������.��̼�������������ʾ��ᷢ����ѧ��Ӧ��ʧЧ�����й�̼����ֻ����ԭ������������.
A��MnO2 B��KMnO4��Һ
C��ϡ���� D��Na2SO3��Һ
��3��Ư���������ƣ�NaClO2���ڳ����ںڰ����ɱ���һ�꣬������ȶ��ɷֽ⣬��Ӧ�����ӷ���ʽΪ��HClO2��ClO2����H����Cl����H2O��δ��ƽ��.��1 mol HClO2�����ֽⷴӦʱ��ת�Ƶĵ��Ӹ�������������.
��1��328 3228��H2O��2��K2Cr2O7��̼Ԫ�ء���3��A����4��6.02��1023
��1��2ClO3����SO��2H��===2ClO2��SO��H2O��2��D����3��4.816��1023

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�