��Ŀ����
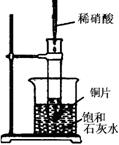
 ��ͼ��ʾ�����Թܷ���ʢ��25����履��ʯ��ˮ���ձ��У��Թ��п�ʼ���뼸��þ�������õιܵ���5mL���������Թ��У��Իش��������⣺����ʾ��Ca��OH��2�ܽ�������¶ȵ����߶���С��
��ͼ��ʾ�����Թܷ���ʢ��25����履��ʯ��ˮ���ձ��У��Թ��п�ʼ���뼸��þ�������õιܵ���5mL���������Թ��У��Իش��������⣺����ʾ��Ca��OH��2�ܽ�������¶ȵ����߶���С����1��ʵ���й۲쵽��������
þ���������ݲ�����þ�����ܽ⣬ʯ��ˮ�����
þ���������ݲ�����þ�����ܽ⣬ʯ��ˮ�����
����2����������ʵ�������ԭ����
Mg+2HCl=MgCl2+H2���÷�Ӧ�Ƿ��ȷ�Ӧ����ϵ�¶�����ʹCa��OH��2�ܽ�Ƚ��Ͷ���������
Mg+2HCl=MgCl2+H2���÷�Ӧ�Ƿ��ȷ�Ӧ����ϵ�¶�����ʹCa��OH��2�ܽ�Ƚ��Ͷ���������
����3��д���йص����ӷ�Ӧ����ʽ
Mg+2H+=Mg2++H2��
Mg+2H+=Mg2++H2��
����4����ʵ���֪��MgCl2��H2��������
��
��
������ڡ�����С�ڡ����ڡ���þ�������������������5���罫�����С�25����履��ʯ��ˮ�����ɡ�20���̼�����ϡ�����̽��ʵ�飬ʵ���й۲쵽����һ������
С�Թ�����ϣ����ձ��ڣ������ݲ���
С�Թ�����ϣ����ձ��ڣ������ݲ���
����ԭ�����¶����߽���CO2�ܽ�ȶ��ų�CO2����
�¶����߽���CO2�ܽ�ȶ��ų�CO2����
����������ͼ��ʾ��ʵ���У����Թ��ڵ���ϡ���ᣬ������þ���ҷ�Ӧ�ų�������ͬʱ��Ӧ�ų�������ʹ����ʯ��ˮ��Һ�¶����ߣ��¶��������������ܽ�ȼ�С��������Һ���������������ƶ�ʹ��Һ����ȥ����ǣ�̼������Һ�в��ȶ��ֽ����ɶ�����̼���壮
����⣺��1��þ��������ҷ�Ӧ���ɹ۲쵽�����������壬��Ӧ�ų�����ʹ������Һ�¶����ߣ������������ʣ��۲쵽��Һ����ǣ�
�ʴ�Ϊ��þƬ���д������ݣ�þƬ���ܽ⣬�ձ�����Һ����ǣ�
��2��þ��������ҷ�Ӧ�������������ų��������ȣ������������Ƶ��ܽ�����¶����߶���С�����Ա���ʯ��ˮ���º���������������ʹ��Һ�ʻ���״��
�ʴ�Ϊ��þ�����ᷴӦ����H2���÷�ӦΪ���ȷ�Ӧ��Ca��OH��2��ˮ���ܽ�����¶����߶���С��
��3��þ�����ᷢ���û���Ӧ�������Ȼ�þ����������Ӧ�Ļ�ѧ����ʽΪMg+2HCl�TMgCl2+H2����
���ӷ���ʽΪ��Mg+2H+=Mg2++H2�����ʴ�Ϊ��Mg+2H+=Mg2++H2����
��4������Ӧ����������������������ʱ����Ӧ�Ƿ��ȷ�Ӧ����MgCl2��Һ��H2��������С��þƬ����������������ʴ�Ϊ��С�ڣ�
��5���罫�����С�25����履��ʯ��ˮ�����ɡ�20���̼�����ϡ�����̽��ʵ�飬С�Թ�����ϣ����ձ��ڣ������ݲ�������Ϊ�¶����߽���CO2�ܽ�ȶ��ų�CO2������¶�����̼��ֽ����CO2���壬
�ʴ�Ϊ������ϣ����ձ��ڣ������ݲ������¶����߽���CO2�ܽ�ȶ��ų�CO2������¶�����̼��ֽ����CO2���壮
�ʴ�Ϊ��þƬ���д������ݣ�þƬ���ܽ⣬�ձ�����Һ����ǣ�
��2��þ��������ҷ�Ӧ�������������ų��������ȣ������������Ƶ��ܽ�����¶����߶���С�����Ա���ʯ��ˮ���º���������������ʹ��Һ�ʻ���״��
�ʴ�Ϊ��þ�����ᷴӦ����H2���÷�ӦΪ���ȷ�Ӧ��Ca��OH��2��ˮ���ܽ�����¶����߶���С��
��3��þ�����ᷢ���û���Ӧ�������Ȼ�þ����������Ӧ�Ļ�ѧ����ʽΪMg+2HCl�TMgCl2+H2����
���ӷ���ʽΪ��Mg+2H+=Mg2++H2�����ʴ�Ϊ��Mg+2H+=Mg2++H2����
��4������Ӧ����������������������ʱ����Ӧ�Ƿ��ȷ�Ӧ����MgCl2��Һ��H2��������С��þƬ����������������ʴ�Ϊ��С�ڣ�
��5���罫�����С�25����履��ʯ��ˮ�����ɡ�20���̼�����ϡ�����̽��ʵ�飬С�Թ�����ϣ����ձ��ڣ������ݲ�������Ϊ�¶����߽���CO2�ܽ�ȶ��ų�CO2������¶�����̼��ֽ����CO2���壬
�ʴ�Ϊ������ϣ����ձ��ڣ������ݲ������¶����߽���CO2�ܽ�ȶ��ų�CO2������¶�����̼��ֽ����CO2���壮
���������⿼���˻�ѧ��Ӧ����������仯������ע�����ͨ����ѧ�仯���ȶ�ʹ������Һ�¶����ߣ��������Ƶı�����Һ�У����������ܽ�����¶����߶���С�ģ����������ͻ����ǽ������Ĺؼ���

��ϰ��ϵ�д�
 �������ͬ����ϰϵ�д�
�������ͬ����ϰϵ�д�
�����Ŀ
 ��ͼ��ʾ�����Թܷ���ʢ25��ʱ����ʯ��ˮ���ձ��У��Թ��п�ʼ���뼸С��þƬ�����õιܵ���5mL�������Թ��У�
��ͼ��ʾ�����Թܷ���ʢ25��ʱ����ʯ��ˮ���ձ��У��Թ��п�ʼ���뼸С��þƬ�����õιܵ���5mL�������Թ��У�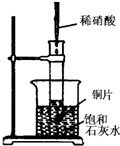 ��ͼ��ʾ�����Թܷ���ʢ��25��ʱ����ʯ��ˮ���ձ��У��Թ��п�ʼ���뼸С��ͭƬ�����õιܵ���10mLϡ���ᣮ�ݴ˻ش��������⣺
��ͼ��ʾ�����Թܷ���ʢ��25��ʱ����ʯ��ˮ���ձ��У��Թ��п�ʼ���뼸С��ͭƬ�����õιܵ���10mLϡ���ᣮ�ݴ˻ش��������⣺