题目内容
燃烧a g CH4气体生成二氧化碳气体和9 g液态水时,放出的热量为222.5 kJ。下列表示CH4燃烧热的热化学方程式为
A、CH4(g)+2O2(g)=CO2(g)+2H2O(l);ΔH=-222.5 kJ/mol
B、CH4(g)+2O2(g)=CO2(g)+2H2O(g);ΔH=-890.0 kJ/mol
C、CH4(g)+2O2(g)=CO2(g)+2H2O(l);ΔH=-890.0 kJ/mol
D、CH4(g)+2O2(g)=CO2(g)+2H2O(l);ΔH=+890.0 kJ/mol
C

练习册系列答案
 应用题作业本系列答案
应用题作业本系列答案
相关题目
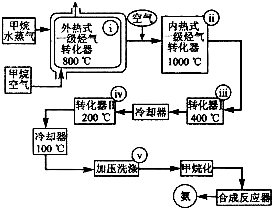 某工厂以天然气、水蒸气和空气为原料和能源合成氨的流程如图.该流程中外热式一级烃转化器系指以CH4为燃料在烃气转化器外面加热使之维持800℃高温的工业装置.内热式一级烃气转 化器系指以H2在装置内燃烧为能量维护一 级烃气转化器所生成的CO与H20(g)反应 生成C02和H2所需1000℃高温的装置.
某工厂以天然气、水蒸气和空气为原料和能源合成氨的流程如图.该流程中外热式一级烃转化器系指以CH4为燃料在烃气转化器外面加热使之维持800℃高温的工业装置.内热式一级烃气转 化器系指以H2在装置内燃烧为能量维护一 级烃气转化器所生成的CO与H20(g)反应 生成C02和H2所需1000℃高温的装置. O2(g)===CO(g)+2H2(g);
O2(g)===CO(g)+2H2(g); CH3OH(g);△H=-Q kJ·mol-1(Q>O),达到平衡后的压强是开始时压强的0.6倍,放出热量Q1kJ。
CH3OH(g);△H=-Q kJ·mol-1(Q>O),达到平衡后的压强是开始时压强的0.6倍,放出热量Q1kJ。
