��Ŀ����
��֪�л���ס��ҡ�����������Ϣ��
|
|
�� |
�� |
�� |
|
����Ԫ�� |
C��H |
C��H��F |
C��H��F |
|
���������� |
26 |
|
|
|
�ṹ�ص� |
������� |
|
|
�ݴ��ƶϣ�
��1���ķ���ʽΪ����������������2����ԭ�ӱ�Fԭ��ȡ�������õ��л�������������������֡�
��2���������������Ļ�����Ʒ���ɴ���ijЩ�ƻ�������ķ�������Ʒ������������������C��H��F��ԭ�Ӹ���֮��Ϊ1��2��2�����ҵĽṹ��ʽΪ�����������������������й����ҵ���������ȷ��������������
A. ����ӹ���Ϊ���������� B. ��������ˮ������Ӧ����ɫ
C. 1 mol���������1 mol F2����ȡ����Ӧ D. ��û��ͬ���칹
��3�����ס��Ұ����ʵ���֮��1��1��ϣ����û�����ƽ��Ħ���������ڱ���Ħ���������������Ӳ�����ͬ���칹�壬����ķ���ʽΪ��������������
��8�֣���1��C3H8��1�֣���4��2�֣�
��2��CH2F2��1�֣���D��2�֣� ��3��C2H5F��2�֣�
��������
�����������1�����DZ��͵��л��������������ͨʽCnH2n��2�����Ը�����6n��2n��2��26�����n��3����ķ���ʽΪC3H8������������2����ԭ�ӱ�Fԭ��ȡ�������õ��л����������4�֣��ֱ���CH3CHFCH2F��CH2CH2CH2F��CHF2CH2CH3��CH3CF2CH3��
��2��������C��H��F��ԭ�Ӹ���֮��Ϊ1��2��2�����ҵĽṹ��ʽΪCH2F2���ҿ��Կ����Ǽ�������е�2����ԭ�ӱ�Fԭ�Ӵ��棬��������ӹ���Ϊ�������ͣ����������������ͣ�A����ȷ��û��̼̼˫��������ˮ����Ӧ��B����ȷ��1 mol���������2 mol F2����ȡ����Ӧ��C����ȷ�����ڼ��������������ͽṹ�����Ըû����ﲻ����ͬ���칹�壬��ѡD��
��3���ס��Ұ����ʵ���֮��1��1��ϣ��������ƽ����Է���������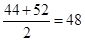 �����ڱ�����Է�������Ҳ��48���ұ�������ͬ���칹�壬����Ļ�ѧʽ��C2H5F��
�����ڱ�����Է�������Ҳ��48���ұ�������ͬ���칹�壬����Ļ�ѧʽ��C2H5F��
���㣺�����л��ﻯѧʽ���ṹ��ʽ�ļ�����ж�
�������������е��Ѷȵ����⣬���������ӱ��������ǿ�����ض�ѧ������֪ʶ�ļ����ѵ����ּ������ѧ�����������ɡ��ܽ����������������������ѧ���������������ͳ���˼ά������

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д���֪�л���ס��ҡ�����������Ϣ��
| | �� | �� | �� |
| ����Ԫ�� | C��H | C��H��F | C��H��F |
| ���������� | 26 | | |
| �ṹ�ص� | �����л��� | | |
��1���ķ���ʽΪ����������������2����ԭ�ӱ�Fԭ��ȡ�������õ��л�������������������֡�
��2���������������Ļ�����Ʒ���ɴ���ijЩ�ƻ�������ķ�������Ʒ������������������C��H��F��ԭ�Ӹ���֮��Ϊ1��2��2�����ҵĽṹ��ʽΪ�����������������������й����ҵ���������ȷ��������������
A. ����ӹ���Ϊ���������� B. ��������ˮ������Ӧ����ɫ
C. 1 mol���������1 mol F2����ȡ����Ӧ D. ��û��ͬ���칹
��3�����ס��Ұ����ʵ���֮��1��1��ϣ����û�����ƽ��Ħ���������ڱ���Ħ���������������Ӳ�����ͬ���칹�壬����ķ���ʽΪ��������������
��֪�л���ס��ҡ�����������Ϣ��
|
|
�� |
�� |
�� |
|
����Ԫ�� |
C��H |
C��H��F |
C��H��F |
|
���������� |
26 |
|
|
|
�ṹ�ص� |
������� |
|
|
�ݴ��ƶϣ�
��1���ķ���ʽΪ����������������2����ԭ�ӱ�Fԭ��ȡ�������õ��л�������������������֡�
��2���������������Ļ�����Ʒ���ɴ���ijЩ�ƻ�������ķ�������Ʒ������������������C��H��F��ԭ�Ӹ���֮��Ϊ1��2��2�����ҵĽṹ��ʽΪ�����������������������й����ҵ���������ȷ��������������
A. ����ӹ���Ϊ���������� B. ��������ˮ������Ӧ����ɫ
C. 1 mol���������1 mol F2����ȡ����Ӧ D. ��û��ͬ���칹
��3�����ס��Ұ����ʵ���֮��1��1��ϣ����û�����ƽ��Ħ���������ڱ���Ħ���������������Ӳ�����ͬ���칹�壬����ķ���ʽΪ��������������
��֪�л���ס��ҡ�����������Ϣ��
| �� | �� | �� | |
| ����Ԫ�� | C��H | C��H��F | C��H��F |
| ���������� | 26 | ||
| �ṹ�ص� | �����л��� |
�ݴ��ƶϣ�
��1���ķ���ʽΪ����������������2����ԭ�ӱ�Fԭ��ȡ�������õ��л�������������������֡�
��2���������������Ļ�����Ʒ���ɴ���ijЩ�ƻ�������ķ�������Ʒ������������������C��H��F��ԭ�Ӹ���֮��Ϊ1��2��2�����ҵĽṹ��ʽΪ�����������������������й����ҵ���������ȷ��������������
A. ����ӹ���Ϊ���������� B. ��������ˮ������Ӧ����ɫ
C. 1 mol���������1 mol F2����ȡ����Ӧ D. ��û��ͬ���칹
��3�����ס��Ұ����ʵ���֮��1��1��ϣ����û�����ƽ��Ħ���������ڱ���Ħ���������������Ӳ�����ͬ���칹�壬����ķ���ʽΪ��������������




 d��
d��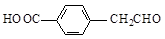
 �ṹ)����X�Ľṹ��ʽΪ
(��дһ��)��
�ṹ)����X�Ľṹ��ʽΪ
(��дһ��)��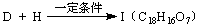 �������������I����
��
�������������I����
��