��Ŀ����
�����±��ṩ���������г��������Լ���ѡ������ʵ����Ӧʵ��Ŀ�ĵ���
| ѡ�� | ʵ��Ŀ�� | ���� |
| A | ��ȥ�������������е���ɳ | ©��������ֽ�����ձ��������� |
| B | ��ʳ��ˮ�л��NaCl���� | ���������������ƾ��ơ������� |
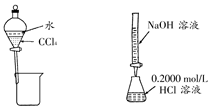
| C | ��0.10mol��L��1������ⶨδ֪Ũ�ȵ�NaOH��ҺŨ�� | ��ʽ�ζ��ܡ���ʽ�ζ��ܡ���ƿ���� ͷ�ιܡ��ձ� |
| D | ��MnO2��Ũ������ȡ������� ��Cl2 | Բ����ƿ����Һ©�����ƾ��ơ�ϴ��ƿ�� ����ƿ�����ܡ�ʯ���� |
B
�������������A����ȥ�������������е���ɳ�ù��˵ķ�������������������ʵ�֣�����B����ʳ��ˮ�л��NaCl�����������ķ�����Ӧ�������������������ʵ�֣���ȷ��C����0.10mol��L��1������ⶨδ֪Ũ�ȵ�NaOH��ҺŨ��������к͵ζ��ķ���������������ʵ�֣�����D����MnO2��Ũ������ȡ���������Cl2������������ʵ�֣�����
���㣺���黯ѧʵ�����������

��ϰ��ϵ�д�
�����Ŀ
����ʵ���ܴﵽ��ӦĿ�ĵ���
| A����ͼ��װ����ȡ���ռ����� | B����ͼ��װ����ȡ���ռ���ϩ |
| C����ͼ��װ���Ʊ����������� | D����ͼ��װ����ȡ�������� |
����˵����ȷ����
| A���������ҵĽ���Һ�м����������ˮ����֤I����ȫ����ΪI2 |
| B����ʳ�����Ậ���IJⶨ�У�û�п��ƺõζ��յ㣬��Һ�����ɫ�����������ʵ�� |
| C����˾ƥ�־�������ѪС���������ã�������������Ѫ�ܼ�����������н�ǿ�����ԣ����Ƴɰ�˾ƥ�ֳ���Ƭ |
| D��Ħ���ε��Ʊ�ʵ���У���FeSO4��(NH4)2SO4���Һ�����������������д����������������ˣ����������ƾ�ϴȥ��������ˮ�� |
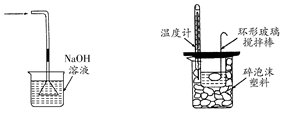
������Լס��ҡ�����������ʵ�鼰װ�õ�˵��������������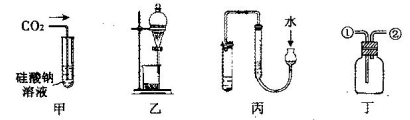
| A��������֤��̼�ķǽ����Աȹ�ǿ |
| B����װ�ÿ����ڵ�ˮ�е����ȡ����Һ |
| C���ñ�ͼʾ�ķ�������װ�õ������� |
| D����װ�ã��Ӣٽ������ռ�NO���Ӣڽ������ռ�NH3 |
����˵������ȷ����
| A���ư�˾ƥ��ʱ��ˮϴ�Ӳ�Ʒ��������Ħ����ʱ�þƾ�ϴ�Ӳ�Ʒ |
| B��ֽ������ͨ���Ѳ���ˮ���ܵ��л��ܼ���Ϊ�̶��� |
| C���к͵ζ�ʵ���У�����ƿ����ƿ������ˮϴ����ʹ�ã��ζ��ܺ���Һ��������ˮϴ������������ϴ��ʹ�� |
| D����ѹ����װ���У�����©���ľ���б��Ӧ������ƿ��֧�ܿ���ԣ��Ա������� |
�������и�ʵ��װ�õ������У���ȷ����(����)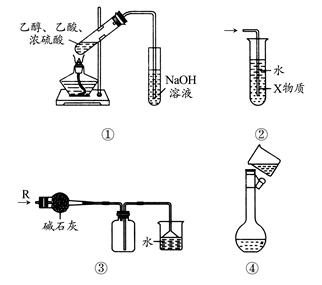
| A��װ�â��Ʊ��������� |
| B��װ�â���X��ΪCCl4������������NH3��HCl������ֹ���� |
| C��װ�âۿ����ڸ���ռ�Cl2�������ն����Cl2 |
| D��װ�â�����һ�����ʵ���Ũ�ȵ���Һʱת��Һ�� |
����ʵ����������ȷ���� (����)��
| A����ʪ���pH��ֽ��ϡ����Һ��pH���ⶨֵƫС |
| B��������ƿ������Һ������ʱ���ӿ̶��ߣ�������ҺŨ��ƫ�� |
| C���ζ�ǰ�ζ����������ݣ��յ����ʱ�����ݣ��������ƫС |
| D���ⶨ�кͷ�Ӧ�ķ�Ӧ��ʱ��������������У������¶�ƫ�� |
�����й�ʵ��IJ�������������ȷ����(����)
| A����������ƽ��ȡ10.5 g NaClʱӦ���ұ������з���10 g���� |
| B�������ᴿʱ,Ϊ�˼ӿ��������,���ò���������������е�Һ�� |
| C����Һ����ʱ,��Һ©�����²�Һ����¿ڷų�,�ϲ�Һ����Ͽڵ��� |
| D���ⶨ��ҺpH�IJ���:��pH��ֽ���ڱ�������,�ò�����պȡ��Һ,����pH��ֽ���в�,Ȼ�������ɫ������ |