��Ŀ����
��ԭ���⣩��8�֣���ӦA(g)+B(g)  C(g)+D(g)�����е������仯��ͼ��ʾ���ش��������⡣
C(g)+D(g)�����е������仯��ͼ��ʾ���ش��������⡣
��1���÷�Ӧ��__________��Ӧ������ȡ������ȡ�����
��2������Ӧ�ﵽƽ��ʱ�������¶ȣ�A��ת����________
���������С�������䡱����
��3���ڷ�Ӧ��ϵ�м����������Ӧ��������E1��E2�ı仯
�ǣ�E1_______,E2_______���������С�������䡱����
��4����֪�����Ȼ�ѧ����ʽ��
��H2��g�� +1/2O2��g����H2O��l������H="-285" kJ��mol-1
��H2��g�� +1/2O2��g����H2O��g������H="-241.8" kJ��mol-1
��C��s�� +1/2O2��g����CO��g������H="-110.5" kJ��mol-1
�� C��s�� +O2��g����CO2��g������H="-393.5" kJ��mol-1
�ش��������⣺
�� ȼ��1gH2����Һ̬ˮ���ų�������Ϊ ��
��д��COȼ���ȵ��Ȼ�ѧ����ʽ ��

����

��ϰ��ϵ�д�
�����Ŀ
 2C(g)+2D(g) ��H=Q��2 minĩ�ﵽƽ�⣬����0.8 mol D��
2C(g)+2D(g) ��H=Q��2 minĩ�ﵽƽ�⣬����0.8 mol D��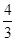 mol����ʹƽ��ʱ�����ʵ����ʵ���Ũ����ԭƽ����ͬ����Ӧ�ü���B ����������mol��
mol����ʹƽ��ʱ�����ʵ����ʵ���Ũ����ԭƽ����ͬ����Ӧ�ü���B ����������mol�� C(g)+D(g)�����е������仯��ͼ��ʾ���ش��������⡣
C(g)+D(g)�����е������仯��ͼ��ʾ���ش��������⡣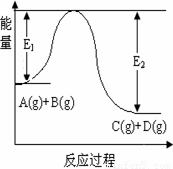
 N2O4(g) ��H<0���ܱ������дﵽƽ�⣬������������ȷ���� �� ��
N2O4(g) ��H<0���ܱ������дﵽƽ�⣬������������ȷ���� �� ��