ΧβΡΩΡΎ»ί
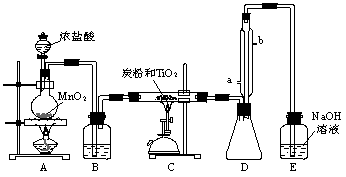 (±ΨΧβΦΤ9Ζ÷)ΥΡ¬»Μ·ν― «Έό…Ϊ“ΚΧεΘ§Ζ–ΒψΈΣ136ΓφΘ§ΥϋΦΪ“ΉΥ°ΫβΘ§”ωΩ’Τχ÷–ΒΡΥ°’τΤχΦ¥≤ζ…ζΓΑΑΉ―ΧΓ±(TiCl4ΘΪH2OΘΫTiOCl2ΘΪ2HClΓϋ)ΓΘTiCl4Ω…”…TiO2”κCl2Ζ¥”Π÷ΤΒΟ(TiO2≤Μ”κHClΖ¥”Π)ΓΘ¥ΥΖ¥”Π‘Ύ1000ΓφΗΏΈ¬œ¬Ϋχ––ΒΡΚή¬ΐΘ§ΒΪ»γΙϊ”–ΧΩΖέ¥φ‘Ύ ±650ΓφΓΪ850ΓφΖ¥”Π±ψΩ…Υ≥άϊΫχ––ΓΘ”“ΆΦ «Ρ≥Ά§―ß…ηΦΤΒΡ Β―ι “÷Τ±ΗTiCl4ΒΡΉΑ÷ΟΓΘ«κΜΊ¥πΘΚ
(±ΨΧβΦΤ9Ζ÷)ΥΡ¬»Μ·ν― «Έό…Ϊ“ΚΧεΘ§Ζ–ΒψΈΣ136ΓφΘ§ΥϋΦΪ“ΉΥ°ΫβΘ§”ωΩ’Τχ÷–ΒΡΥ°’τΤχΦ¥≤ζ…ζΓΑΑΉ―ΧΓ±(TiCl4ΘΪH2OΘΫTiOCl2ΘΪ2HClΓϋ)ΓΘTiCl4Ω…”…TiO2”κCl2Ζ¥”Π÷ΤΒΟ(TiO2≤Μ”κHClΖ¥”Π)ΓΘ¥ΥΖ¥”Π‘Ύ1000ΓφΗΏΈ¬œ¬Ϋχ––ΒΡΚή¬ΐΘ§ΒΪ»γΙϊ”–ΧΩΖέ¥φ‘Ύ ±650ΓφΓΪ850ΓφΖ¥”Π±ψΩ…Υ≥άϊΫχ––ΓΘ”“ΆΦ «Ρ≥Ά§―ß…ηΦΤΒΡ Β―ι “÷Τ±ΗTiCl4ΒΡΉΑ÷ΟΓΘ«κΜΊ¥πΘΚ
(1)AΉΑ÷Ο÷–Ζ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣ___________________________________________ΓΘ
(2)BΉΑ÷Ο÷–ΒΡ ‘ΦΝΈΣ________________Θ§Ής”Ο «______________________________ΓΘ
(3) Β―ι÷–ΧΩΖέΒΡΉς”ΟΈΣ_______________________ΓΘ
(4)DΉΑ÷Ο÷–άδΡΐΥ°ΒΡΥ°ΝςΖΫœρΈΣ____________Ϋχ____________≥ωΘ§ΗΟΉΑ÷ΟΒΡΉς”Ο «_________________ΓΘ
(5)EΉΑ÷Ο÷–NaOH»ή“ΚΒΡΉς”Ο «_______________________________________ΓΘ
(6)ΗΟ…ηΦΤΖΫΑΗ÷–≥ΐΖ÷“Κ¬©ΕΖ÷–ΒΡ≈®―ΈΥα≤ΜΡήΥ≥άϊΒΈœ¬ΓΔE≤ΜΡήΖβΩΎΚΆΩ…ΡήΒΙΈϋΆβΘ§ΜΙ”–“Μ¥ΠΟςœ‘ΒΡ≤ΜΉψ «_______________________________________________________ΓΘ
(1)MnO2ΘΪ4HCl(≈®)![]() MnCl2ΘΪ2H2OΘΪCl2Γϋ(2Ζ÷)
MnCl2ΘΪ2H2OΘΪCl2Γϋ(2Ζ÷)
(2)≈®ΝρΥαΘΜΗ…‘οCl2ΘΜ
(3)¥ΏΜ·Ής”Ο(ΟΩΩ’1Ζ÷)
(4)aΘΜb(1Ζ÷)ΘΜάδΡΐΜΊΝςTiCl4(1Ζ÷)
(5)Έϋ ’Έ¥Ζ¥”ΠΒΡCl2Θ§Ζά÷ΙΈέ»ΨΜΖΨ≥(1Ζ÷)
(6)DΓΔE÷°ΦδΈόΗ…‘οΉΑ÷Ο(1Ζ÷)

 ‘ΡΕΝΩλ≥ΒœΒΝ–¥πΑΗ
‘ΡΕΝΩλ≥ΒœΒΝ–¥πΑΗ