��Ŀ����
����Ŀ����������(NaNO2)��һ�ֹ�ҵ�Σ������ʳ�����ơ�������ijѧϰС����Ƶ�NaNO2��ȡʵ��ʹ��ȼ���ʵ�顣��С���ռ���������ϣ�
��SO2��HNO3��Һ��Ӧ����NOx��H2SO4
��3NO2-+2H+=2NO��+NO3-+H2O
��NO2-+Ag+=AgNO2��(AgNO2Ϊ����ɫ�ӽ���ɫ���壬��ˮ���γɳ���)
���������Ƶ���ȡʵ��
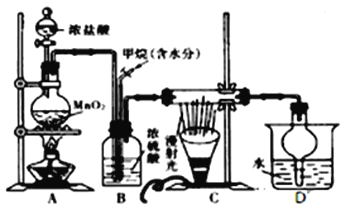
��1������a������Ϊ________________________��Aװ���з����Ļ�ѧ��Ӧ����ʽΪ_____________________________________________��
��2��Bװ���ж�����ݵ�������_________________________________________________��
��3����װ��B���ݳ���NO��NO2�������ʵ���֮��Ϊ2��1����װ��B�з�����Ӧ�Ļ�ѧ����ʽΪ_________________________________________________________��
��4��ʵ������������Cװ������ҺpH>7������C�����ɵ�NaNO2�IJ������½���������_____________________________________________________��
��5����������������Ϣ���ʵ��֤��Cװ������NO2������_________________________________��(��ѡ�õ��Լ���ϡ���ᡢ��������Һ��NaOH��Һ)
���������ƵĴ��ȼ���
��֪��NO2-+MnO4-+H+��NO3-+Mn2++H2O
��6����Ӧ������C����Һͨ���ᾧ���NaNO2�ֲ�Ʒmg,�ܽ��ϡ����250mL���ֱ�ȡ25.00mL��cmol/L������KMnO4��Һƽ�еζ����Σ�ƽ��ÿ����������KMnO4��Һ�����ΪVmL����ֲ�Ʒ��NaNO2����������Ϊ_____________(�ú�c��V��m��ʽ�ӱ�ʾ)��
���𰸡� ��Һ©�� Na2SO3+H2SO4(Ũ)=Na2SO4+SO2��+H2O ������������Һ�ĽӴ�������÷�Ӧ��ֽ��� 7SO2+6HNO3+4H2O=4NO+2NO2+7H2SO4 ���pH<7,�������λ�ת��Ϊ�����κ�NO���壬ʹ������½� ȡC����Һ�������Թ��У�������ϡ�������������ɣ������������ɫ����֤��C����NO2-���� (345cv/2m)%��3.45cv/2m
����������1������a������Ϊ��Һ©����Aװ��������Ũ�������������Ʒ�Ӧ���������ơ����������ˮ�Ʊ������������Ļ�ѧ��Ӧ����ʽΪNa2SO3+H2SO4(Ũ)=Na2SO4+SO2��+H2O����2��Bװ���ж�����ݵ�������������������Һ�ĽӴ�������÷�Ӧ��ֽ��У���3����װ��B���ݳ���NO��NO2�������ʵ���֮��Ϊ2��1����װ��B�з�����Ӧ�Ļ�ѧ����ʽΪ7SO2+6HNO3+4H2O=4NO+2NO2+7H2SO4����4��������֪��Ϣ��֪���pH<7,�������λ�ת��Ϊ�����κ�NO���壬ʹNaNO2�IJ������½�����5��ȡC����Һ�������Թ��У�������ϡ�������������ɣ������������ɫ����֤��C����NO2-���ɣ���6����������KMnO4��0.001cVmol�����ݷ���ʽ��֪����������0.0025cVmol����˴ֲ�Ʒ��NaNO2����������Ϊ![]() ��
��

����Ŀ����ˮAlCl3��һ����Ҫ�Ļ���ԭ�ϡ�ij����С�鳢����ȡ��ˮAlCl3���������̽����
������Ϣ:��ˮAlCl3��178�����������׳��⣬������ʪ�����������ɫ������
��̽��һ����ˮAlCl3��ʵ�����Ʊ�
������ͼװ�ã��ø�������������ڼ����������봿���۷�Ӧ��ȡ��ˮAlCl3����ѡ���ҩƷ:��������Ũ������ϡ����������ʳ��ˮ���������̷�ĩ����ˮ�Ȼ�����ϡ������Ũ����������������Һ��

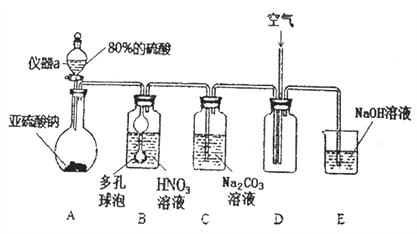
(1)д��װ��A�����ķ�Ӧ����ʽ__________��
(2)װ��E���õ�������ѡҩƷ�е�________(���������)��װ��F��������__________��
(3)д����ˮAlCl3������ʪ����������Ӧ�Ļ�ѧ����ʽ__________��
��̽����������Ũ�ȶ������Ʊ���Ӱ��
̽���������̷�ĩ��Ũ����ķ�Ӧ���������Ũ�Ƚ��ͣ���Ӧ��ֹͣ��ԭ��:
(4)�������:����1.Cl-Ũ�Ƚ���Ӱ�����������ɣ�����2.__________��
(5)���ʵ�鷽��:(��ѡ�Լ�:ŨH2SO4��NaCl���塢MnO2���塢ϡ����)
���� | ʵ����� | Ԥ������ͽ��� |
�� | �����ٲ���������װ���У�����_____�������� | ���л���ɫ�������ɣ������1���� |
�� | __________ | ���л���ɫ�������ɣ������2���� |
��̽��������ˮAlCl3�ĺ����ⶨ���������
ȡD�з�Ӧ�����ù���2.0g������������������Һ��Ӧ���ⶨ������������(���������ɱ�״��)���ظ��ⶨ���Σ���������:
��һ��ʵ�� | �ڶ���ʵ�� | ������ʵ�� | |
D������� | 2.0g | 2.0g | 2.0g |
��������� | 334.5mL | 336.0mL | 337.5mL |
(6)���ݱ������ݣ��������ù�������ˮAlCl3����������_________��
(7)������ΪD���Ƶ���ˮAlCl3����������ƫ�ͣ����ܵ�һ��ԭ����__________��
����Ŀ���״��ɲ��ö��ַ����Ʊ�������;�㷺������Ҫ�Ļ���ԭ�ϡ�
�����úϳ�����CO��CO2��H2���ڴ��������ºϳɼ״���������Ӧ���£�
��CO(g)+2H2(g)![]() CH3OH(g)
CH3OH(g)
��CO2(g)+3H2(g)![]() CH3OH(g)+H2O(g)
CH3OH(g)+H2O(g)
��1����Ӧ�������������仯����ͼ��ʾ������˵����ȷ����______________(��ѡ����)��

A������Ӧ����H=�淴Ӧ���-����Ӧ���
B����1molCO(g)��2molH2(g)�����ܱ������г�ַ�Ӧ����ƽ��ʱ�ų�������Ϊ91kJ
C����ͼ����Ϣ��֪��������ܸı䷴Ӧ���̺���ЧӦ
D����ͬ�����£�CO(g)��H2(g)��Ӧ����1molҺ̬CH3OH�ų�����������91kJ
��2��������������������Ӧ�ϳ�CH3OH����֪CO��ʹ��Ӧ�Ĵ��������½���
����̼�ȱ�ʾΪf= [(n(H2)-n(CO2)]/[(n(CO)-n(CO2)],��������f=______________ʱ��ԭ��������������ߡ������������������Ը��ڸ�ֵ����̼�ȣ���������______________________________��
���״���������ȡ����������䷴Ӧ����ʽΪ��CH3OH(g)+CO(g)![]() HCOOCH3(g)��H<0��������Ա�IJ����о�������£�
HCOOCH3(g)��H<0��������Ա�IJ����о�������£�
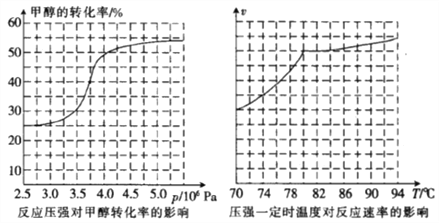
��3���ӷ�Ӧѹǿ�Լ״�ת���ʵ�Ӱ����Ч����ͼ�������ɱ��Ƕȷ�������ҵ��ȡ�������Ӧѡ������ѹǿ��______________________(����3.5��106Pa����4.0��106Pa������5.0��106Pa��)��
��4��ʵ�ʹ�ҵ�����в��õ��¶���80��,��������_______________________________________��
���״����������ںϳɶ����ѣ������ķ�ӦΪ2CH3OH(g)![]() CH3OCH3(g)+H2O(g)
CH3OCH3(g)+H2O(g)
��֪�÷�Ӧ��ij�¶��µ�ƽ�ⳣ��Ϊ900,���¶��£����ܱ������м���CH3OH,��Ӧ��ijʱ�̲�ø����Ũ�����£�
���� | CH3OH | CH3OCH3 | H2O |
Ũ��(mol/L) | 1.25 | 0.9 | 0.9 |
��5���Ƚϴ�ʱ�����淴Ӧ���ʵĴ�С��v��___________v��(����>��<������=��)��
��6��������CH3OH��6min��Ӧ�ﵽƽ�⣬���ʱ����ƽ����Ӧ����v(CH3OH)=___________mol/(L��min)��