��Ŀ����
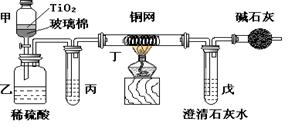
(12�֣�AΪ���������������ռ����������Ϊ88.39����A��Mn2����HNO3��Һ��Ӧ����Һ��Ϊ��ɫ��A��Mn2����������Һ��Ӧ��������ɫ���ɣ����л���ɫ����ų�������Һ�����������������壬��֪0.234g��A��2mol/L�����ᷴӦ����������0.278g��ͬʱ�ų���������373K��1.013��105Paʱ�����Ϊ2.14L����A��������Һ�м���CrO42�����л�ɫ�������ɡ�ͨ����صļ��㣬�ش��������⣺
1��д��A�Ļ�ѧʽ��
2����������ɡ�
3��д�����漰��Ӧ�Ļ�ѧ����ʽ��
1��PbO1.7��6�֣�
2��ÿĦ����PbO1.7�У���0.3mol��PbO��0.7mol��PbO2��1�֣�
3��5PbO2��2Mn2����4H����5Pb2����2MnO4����2H2O��2�֣�
PbO2��4HCl��PbCl2��Cl2��2H2O��1�֣�
PbO��2HCl��PbCl2��H2O��1�֣�
Pb2����CrO42����PbCrO4��1�֣�

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ


 Cr2O72-+H2O������������+6�۸����������ǿ�����ԣ����Խ�C2H5OH����ΪCH3COOH����������ԭΪCr3+��Cr3+��ˮ��Һ�гʻ���ɫ��
Cr2O72-+H2O������������+6�۸����������ǿ�����ԣ����Խ�C2H5OH����ΪCH3COOH����������ԭΪCr3+��Cr3+��ˮ��Һ�гʻ���ɫ��