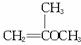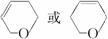��Ŀ����
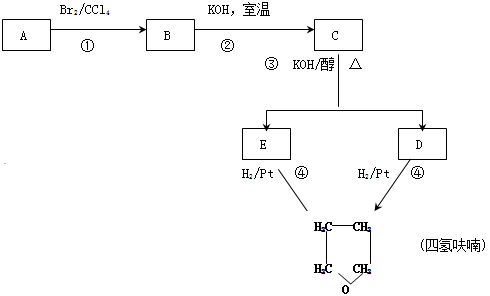
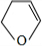
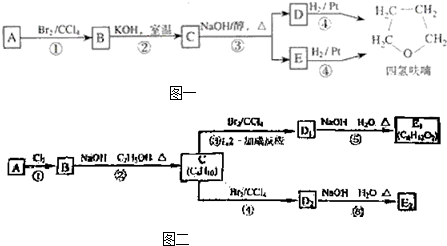
������£�±�����봼��Ӧ�����ѣ�R-O-R�䣩��R-X+R��OH R-O-R��+HX������A�������IJ���Ӧ�ɵõ������ܼ����� ૣ���Ӧ��ͼ��ͼһ��ʾ��
R-O-R��+HX������A�������IJ���Ӧ�ɵõ������ܼ����� ૣ���Ӧ��ͼ��ͼһ��ʾ��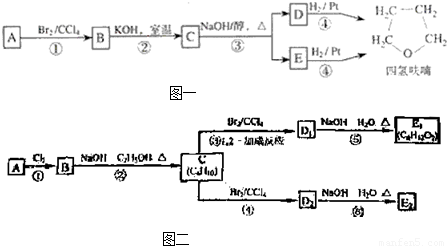
��ش��������⣺
��1��1mol A��1mol H2��һ��������ǡ�÷�Ӧ�����ɱ���һԪ��Y��Y��̼Ԫ�ص���������ԼΪ65%����Y�ķ���ʽΪ ��A���������������ŵ������� ��A�Ľṹ��ʽΪ ��
��2���ڢ٢ڲ���Ӧ���ͷֱ�Ϊ�� ���� ��
��3��������B���еĻ�ѧ���ʣ���д��ĸ���ţ��� ��
a���ɷ���������Ӧ b��ǿ���ǿ�������¾��ɷ�����ȥ��Ӧ
c���ɷ���������Ӧ d���������¿ɷ����Ӿ۷�Ӧ
��4��д��C��D��E�Ľṹ��ʽ��C ��D ��E ��
��5��д��������C��NaOHˮ��Һ��Ӧ�Ļ�ѧ����ʽ�� ��
��6��д���������״���������ͬ���칹��Ľṹ��ʽ�� ��
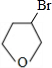
��ij�������A������ͼ��������Է�������Ϊ84��������ױ��������к���̼̼˫�����˴Ź������ױ���������ֻ��һ�����͵��⣮
��1��A�Ľṹ��ʽΪ ��
��2��A��̼ԭ���Ƿ���ͬһƽ�棿 ����ǡ����ǡ�����
��3����ͼ���У�D1��D2��Ϊͬ���칹�壬E1��E2��Ϊͬ���칹�壮
��Ӧ�ڵĻ�ѧ����ʽΪ ��C �Ļ�ѧ������ ��E2�Ľṹ��ʽ�� ���ܡ��ķ�Ӧ���������� �� ��
���𰸡���������YΪ����һԪ�����������ʽΪCnH2n+2O��������C����������Ϊ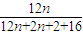 ×100%=65%����n=4����Y�ķ���ʽΪC4H10O��1molA��1molH2ǡ�÷�Ӧ����A�ܺ�Br2/CCl4��Ӧ��˵��A�����к���C=C������A�л�����-OH������Ϣ±�����봼��Ӧ�����ѣ��������Ľṹ����֪AΪCH2=CHCH2CH2OH����BΪCH2BrCHBrCH2CH2OH��CΪ
×100%=65%����n=4����Y�ķ���ʽΪC4H10O��1molA��1molH2ǡ�÷�Ӧ����A�ܺ�Br2/CCl4��Ӧ��˵��A�����к���C=C������A�л�����-OH������Ϣ±�����봼��Ӧ�����ѣ��������Ľṹ����֪AΪCH2=CHCH2CH2OH����BΪCH2BrCHBrCH2CH2OH��CΪ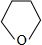
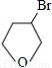 ��D��EΪ
��D��EΪ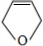 ��
�� ��
��
��ij�������A������ͼ��������Է�������Ϊ84�������ΪCxHy����x���ֵΪ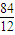 =7��������ױ��������к���̼̼˫��������AΪϩ��������x=6��y=12�����A�Ļ�ѧʽΪC6H12���˴Ź������ױ���������ֻ��һ�����͵��⣬��A�Ľṹ��ʽΪ����CH3��2C=C��CH3��2��A��������Ӧ����B��BΪ����CH3��2C��Cl��C��Cl����CH3��2��B���������ơ��Ҵ������¼��ȣ�������ȥ��Ӧ����C��CΪCH2=C��CH3��-C��CH3��=CH2��C���巢��1��2-�ӳ�����D1����D1ΪCH2BrCBr��CH3��-C��CH3��=CH2��D1����������ˮ��Һ�з���ˮ�ⷴӦ����E1��E1ΪHOCH2C=CCH2OH��D1��D2��Ϊͬ���칹�壬�ʷ�Ӧ�ܷ���1��4-�ӳɣ�D2ΪCH2BrC��CH3��=C��CH3��CH2Br��D2����������ˮ��Һ�з���ˮ�ⷴӦ����E2��E2ΪHOCH2C��CH3��=C��CH3��CH2OH��
=7��������ױ��������к���̼̼˫��������AΪϩ��������x=6��y=12�����A�Ļ�ѧʽΪC6H12���˴Ź������ױ���������ֻ��һ�����͵��⣬��A�Ľṹ��ʽΪ����CH3��2C=C��CH3��2��A��������Ӧ����B��BΪ����CH3��2C��Cl��C��Cl����CH3��2��B���������ơ��Ҵ������¼��ȣ�������ȥ��Ӧ����C��CΪCH2=C��CH3��-C��CH3��=CH2��C���巢��1��2-�ӳ�����D1����D1ΪCH2BrCBr��CH3��-C��CH3��=CH2��D1����������ˮ��Һ�з���ˮ�ⷴӦ����E1��E1ΪHOCH2C=CCH2OH��D1��D2��Ϊͬ���칹�壬�ʷ�Ӧ�ܷ���1��4-�ӳɣ�D2ΪCH2BrC��CH3��=C��CH3��CH2Br��D2����������ˮ��Һ�з���ˮ�ⷴӦ����E2��E2ΪHOCH2C��CH3��=C��CH3��CH2OH��
����⣺��YΪ����һԪ�����������ʽΪCnH2n+2O��������C����������Ϊ ×100%=65%����n=4����Y�ķ���ʽΪC4H10O��1molA��1molH2ǡ�÷�Ӧ����A�ܺ�Br2/CCl4��Ӧ��˵��A�����к���C=C������A�л�����-OH������Ϣ±�����봼��Ӧ�����ѣ��������Ľṹ����֪AΪCH2=CHCH2CH2OH����BΪCH2BrCHBrCH2CH2OH��CΪ
×100%=65%����n=4����Y�ķ���ʽΪC4H10O��1molA��1molH2ǡ�÷�Ӧ����A�ܺ�Br2/CCl4��Ӧ��˵��A�����к���C=C������A�л�����-OH������Ϣ±�����봼��Ӧ�����ѣ��������Ľṹ����֪AΪCH2=CHCH2CH2OH����BΪCH2BrCHBrCH2CH2OH��CΪ ��D��EΪ
��D��EΪ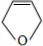 ��
��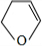 ��
��
��1��������������֪��Y����ʽΪC4H10O��AΪCH2=CHCH2CH2OH������̼̼˫�����ǻ���
�ʴ�Ϊ��C4H10O���ǻ���̼̼˫����CH2=CHCH2CH2OH��
��2����Ӧ����CH2=CHCH2CH2OH����ˮ�����ӳɷ�Ӧ����Ӧ����CH2BrCHBrCH2CH2OH����ȡ����Ӧ����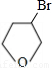 ��
��
�ʴ�Ϊ���ӳɷ�Ӧ��ȡ����Ӧ��
��3��BΪCH2BrCHBrCH2CH2OH������-OH���Է���������Ӧ��������ǿ�ᣨŨH2SO4�������·�����ȥ��Ӧ�����Է���������Ӧ������-Br������ǿ�NaOH�������·�����ȥ��Ӧ�����ܷ����Ӿ۷�Ӧ���ʴ�Ϊ��abc��
��4��������������֪��CΪ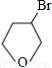 ��D��EΪ
��D��EΪ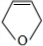 ��
��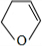 ��
��
�ʴ�Ϊ��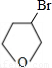 ��
��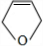 ��
��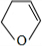 ��
��
��5��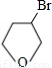 ��NaOHˮ��Һ��Ӧ�Ļ�ѧ����ʽ��
��NaOHˮ��Һ��Ӧ�Ļ�ѧ����ʽ��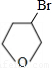 +NaOH
+NaOH
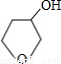 +NaBr��
+NaBr��
�ʴ�Ϊ��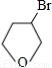 +NaOH
+NaOH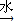
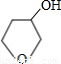 +NaBr����
+NaBr����
��6���������״�����ͬ���칹��Ľṹ��ʽ��CH2=CHOCH2CH3��CH2=CHCH2OCH3��CH3CH=CHOCH3��CH2=C��CH3��OCH3��
�ʴ�Ϊ��CH2=CHOCH2CH3��CH2=CHCH2OCH3��CH3CH=CHOCH3��CH2=C��CH3��OCH3��
��ij�������A������ͼ��������Է�������Ϊ84�������ΪCxHy����x���ֵΪ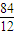 =7��������ױ��������к���̼̼˫��������AΪϩ��������x=6��y=12�����A�Ļ�ѧʽΪC6H12���˴Ź������ױ���������ֻ��һ�����͵��⣬��A�Ľṹ��ʽΪ����CH3��2C=C��CH3��2��A��������Ӧ����B��BΪ����CH3��2C��Cl��C��Cl����CH3��2��B���������ơ��Ҵ������¼��ȣ�������ȥ��Ӧ����C��CΪCH2=C��CH3��-C��CH3��=CH2��C���巢��1��2-�ӳ�����D1����D1ΪCH2Br-CBr��CH3��-C��CH3��=CH2��D1����������ˮ��Һ�з���ˮ�ⷴӦ����E1��E1ΪHOCH2C=CCH2OH��D1��D2��Ϊͬ���칹�壬�ʷ�Ӧ�ܷ���1��4-�ӳɣ�D2ΪCH2BrC��CH3��=C��CH3��CH2Br��D2����������ˮ��Һ�з���ˮ�ⷴӦ����E2��E2ΪHOCH2C��CH3��=C��CH3��CH2OH��
=7��������ױ��������к���̼̼˫��������AΪϩ��������x=6��y=12�����A�Ļ�ѧʽΪC6H12���˴Ź������ױ���������ֻ��һ�����͵��⣬��A�Ľṹ��ʽΪ����CH3��2C=C��CH3��2��A��������Ӧ����B��BΪ����CH3��2C��Cl��C��Cl����CH3��2��B���������ơ��Ҵ������¼��ȣ�������ȥ��Ӧ����C��CΪCH2=C��CH3��-C��CH3��=CH2��C���巢��1��2-�ӳ�����D1����D1ΪCH2Br-CBr��CH3��-C��CH3��=CH2��D1����������ˮ��Һ�з���ˮ�ⷴӦ����E1��E1ΪHOCH2C=CCH2OH��D1��D2��Ϊͬ���칹�壬�ʷ�Ӧ�ܷ���1��4-�ӳɣ�D2ΪCH2BrC��CH3��=C��CH3��CH2Br��D2����������ˮ��Һ�з���ˮ�ⷴӦ����E2��E2ΪHOCH2C��CH3��=C��CH3��CH2OH��
��1��������������֪��A�Ľṹ��ʽΪ����CH3��2C=C��CH3��2���ʴ�Ϊ����CH3��2C=C��CH3��2��
��2����CH3��2C=C��CH3��2�к���C=C˫����ƽ��ṹ��4������Cԭ�Ӵ���C=C˫����ƽ��ṹ�ڣ�����̼ԭ�Ӷ�����ͬһƽ�棬�ʴ�Ϊ���ǣ�
��3����Ӧ���ǣ�CH3��2C��Cl��C��Cl����CH3��2�����������ƴ���Һ�����������·�����ȥ��Ӧ������CH2=C��CH3��-C��CH3��=CH2���÷�Ӧ�Ļ�ѧ����ʽΪ��CH3��2C��Cl��C��Cl����CH3��2+2NaOH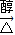 CH2=C��CH3��-C��CH3��=CH2+2NaCl+2H2O��
CH2=C��CH3��-C��CH3��=CH2+2NaCl+2H2O��
CΪCH2=C��CH3��-C��CH3��=CH2���Ļ�ѧ������2��3-����-1��3-����ϩ��
E2�Ľṹ��ʽ��HOCH2C��CH3��=C��CH3��CH2OH��
��Ӧ���Ǽӳɷ�Ӧ����Ӧ����ȡ����Ӧ��
�ʴ�Ϊ����CH3��2C��Cl��C��Cl����CH3��2+2NaOH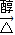 CH2=C��CH3��-C��CH3��=CH2+2NaCl+2H2O��2��3-����-1��3-����ϩ��HOCH2C��CH3��=C��CH3��CH2OH���ӳɷ�Ӧ��ȡ����Ӧ��
CH2=C��CH3��-C��CH3��=CH2+2NaCl+2H2O��2��3-����-1��3-����ϩ��HOCH2C��CH3��=C��CH3��CH2OH���ӳɷ�Ӧ��ȡ����Ӧ��
���������⿼���л����ƶϣ��漰±������ϩ�����ȵ������Լ�����ʽ����⡢ͬ���칹�塢�л���ѧ��Ӧ���ͺͷ���ʽ����д�ȣ���Ŀ�ۺ��Խϴ�ע���ϩ���ļӳɷ�Ӧ���Ѷ��еȣ�
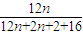 ×100%=65%����n=4����Y�ķ���ʽΪC4H10O��1molA��1molH2ǡ�÷�Ӧ����A�ܺ�Br2/CCl4��Ӧ��˵��A�����к���C=C������A�л�����-OH������Ϣ±�����봼��Ӧ�����ѣ��������Ľṹ����֪AΪCH2=CHCH2CH2OH����BΪCH2BrCHBrCH2CH2OH��CΪ
×100%=65%����n=4����Y�ķ���ʽΪC4H10O��1molA��1molH2ǡ�÷�Ӧ����A�ܺ�Br2/CCl4��Ӧ��˵��A�����к���C=C������A�л�����-OH������Ϣ±�����봼��Ӧ�����ѣ��������Ľṹ����֪AΪCH2=CHCH2CH2OH����BΪCH2BrCHBrCH2CH2OH��CΪ
 ��D��EΪ
��D��EΪ ��
�� ��
����ij�������A������ͼ��������Է�������Ϊ84�������ΪCxHy����x���ֵΪ
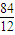 =7��������ױ��������к���̼̼˫��������AΪϩ��������x=6��y=12�����A�Ļ�ѧʽΪC6H12���˴Ź������ױ���������ֻ��һ�����͵��⣬��A�Ľṹ��ʽΪ����CH3��2C=C��CH3��2��A��������Ӧ����B��BΪ����CH3��2C��Cl��C��Cl����CH3��2��B���������ơ��Ҵ������¼��ȣ�������ȥ��Ӧ����C��CΪCH2=C��CH3��-C��CH3��=CH2��C���巢��1��2-�ӳ�����D1����D1ΪCH2BrCBr��CH3��-C��CH3��=CH2��D1����������ˮ��Һ�з���ˮ�ⷴӦ����E1��E1ΪHOCH2C=CCH2OH��D1��D2��Ϊͬ���칹�壬�ʷ�Ӧ�ܷ���1��4-�ӳɣ�D2ΪCH2BrC��CH3��=C��CH3��CH2Br��D2����������ˮ��Һ�з���ˮ�ⷴӦ����E2��E2ΪHOCH2C��CH3��=C��CH3��CH2OH��
=7��������ױ��������к���̼̼˫��������AΪϩ��������x=6��y=12�����A�Ļ�ѧʽΪC6H12���˴Ź������ױ���������ֻ��һ�����͵��⣬��A�Ľṹ��ʽΪ����CH3��2C=C��CH3��2��A��������Ӧ����B��BΪ����CH3��2C��Cl��C��Cl����CH3��2��B���������ơ��Ҵ������¼��ȣ�������ȥ��Ӧ����C��CΪCH2=C��CH3��-C��CH3��=CH2��C���巢��1��2-�ӳ�����D1����D1ΪCH2BrCBr��CH3��-C��CH3��=CH2��D1����������ˮ��Һ�з���ˮ�ⷴӦ����E1��E1ΪHOCH2C=CCH2OH��D1��D2��Ϊͬ���칹�壬�ʷ�Ӧ�ܷ���1��4-�ӳɣ�D2ΪCH2BrC��CH3��=C��CH3��CH2Br��D2����������ˮ��Һ�з���ˮ�ⷴӦ����E2��E2ΪHOCH2C��CH3��=C��CH3��CH2OH������⣺��YΪ����һԪ�����������ʽΪCnH2n+2O��������C����������Ϊ
 ×100%=65%����n=4����Y�ķ���ʽΪC4H10O��1molA��1molH2ǡ�÷�Ӧ����A�ܺ�Br2/CCl4��Ӧ��˵��A�����к���C=C������A�л�����-OH������Ϣ±�����봼��Ӧ�����ѣ��������Ľṹ����֪AΪCH2=CHCH2CH2OH����BΪCH2BrCHBrCH2CH2OH��CΪ
×100%=65%����n=4����Y�ķ���ʽΪC4H10O��1molA��1molH2ǡ�÷�Ӧ����A�ܺ�Br2/CCl4��Ӧ��˵��A�����к���C=C������A�л�����-OH������Ϣ±�����봼��Ӧ�����ѣ��������Ľṹ����֪AΪCH2=CHCH2CH2OH����BΪCH2BrCHBrCH2CH2OH��CΪ ��D��EΪ
��D��EΪ ��
�� ��
����1��������������֪��Y����ʽΪC4H10O��AΪCH2=CHCH2CH2OH������̼̼˫�����ǻ���
�ʴ�Ϊ��C4H10O���ǻ���̼̼˫����CH2=CHCH2CH2OH��
��2����Ӧ����CH2=CHCH2CH2OH����ˮ�����ӳɷ�Ӧ����Ӧ����CH2BrCHBrCH2CH2OH����ȡ����Ӧ����
 ��
���ʴ�Ϊ���ӳɷ�Ӧ��ȡ����Ӧ��
��3��BΪCH2BrCHBrCH2CH2OH������-OH���Է���������Ӧ��������ǿ�ᣨŨH2SO4�������·�����ȥ��Ӧ�����Է���������Ӧ������-Br������ǿ�NaOH�������·�����ȥ��Ӧ�����ܷ����Ӿ۷�Ӧ���ʴ�Ϊ��abc��
��4��������������֪��CΪ
 ��D��EΪ
��D��EΪ ��
�� ��
���ʴ�Ϊ��
 ��
�� ��
�� ��
����5��
 ��NaOHˮ��Һ��Ӧ�Ļ�ѧ����ʽ��
��NaOHˮ��Һ��Ӧ�Ļ�ѧ����ʽ�� +NaOH
+NaOH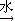
 +NaBr��
+NaBr���ʴ�Ϊ��
 +NaOH
+NaOH
 +NaBr����
+NaBr������6���������״�����ͬ���칹��Ľṹ��ʽ��CH2=CHOCH2CH3��CH2=CHCH2OCH3��CH3CH=CHOCH3��CH2=C��CH3��OCH3��
�ʴ�Ϊ��CH2=CHOCH2CH3��CH2=CHCH2OCH3��CH3CH=CHOCH3��CH2=C��CH3��OCH3��
��ij�������A������ͼ��������Է�������Ϊ84�������ΪCxHy����x���ֵΪ
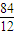 =7��������ױ��������к���̼̼˫��������AΪϩ��������x=6��y=12�����A�Ļ�ѧʽΪC6H12���˴Ź������ױ���������ֻ��һ�����͵��⣬��A�Ľṹ��ʽΪ����CH3��2C=C��CH3��2��A��������Ӧ����B��BΪ����CH3��2C��Cl��C��Cl����CH3��2��B���������ơ��Ҵ������¼��ȣ�������ȥ��Ӧ����C��CΪCH2=C��CH3��-C��CH3��=CH2��C���巢��1��2-�ӳ�����D1����D1ΪCH2Br-CBr��CH3��-C��CH3��=CH2��D1����������ˮ��Һ�з���ˮ�ⷴӦ����E1��E1ΪHOCH2C=CCH2OH��D1��D2��Ϊͬ���칹�壬�ʷ�Ӧ�ܷ���1��4-�ӳɣ�D2ΪCH2BrC��CH3��=C��CH3��CH2Br��D2����������ˮ��Һ�з���ˮ�ⷴӦ����E2��E2ΪHOCH2C��CH3��=C��CH3��CH2OH��
=7��������ױ��������к���̼̼˫��������AΪϩ��������x=6��y=12�����A�Ļ�ѧʽΪC6H12���˴Ź������ױ���������ֻ��һ�����͵��⣬��A�Ľṹ��ʽΪ����CH3��2C=C��CH3��2��A��������Ӧ����B��BΪ����CH3��2C��Cl��C��Cl����CH3��2��B���������ơ��Ҵ������¼��ȣ�������ȥ��Ӧ����C��CΪCH2=C��CH3��-C��CH3��=CH2��C���巢��1��2-�ӳ�����D1����D1ΪCH2Br-CBr��CH3��-C��CH3��=CH2��D1����������ˮ��Һ�з���ˮ�ⷴӦ����E1��E1ΪHOCH2C=CCH2OH��D1��D2��Ϊͬ���칹�壬�ʷ�Ӧ�ܷ���1��4-�ӳɣ�D2ΪCH2BrC��CH3��=C��CH3��CH2Br��D2����������ˮ��Һ�з���ˮ�ⷴӦ����E2��E2ΪHOCH2C��CH3��=C��CH3��CH2OH����1��������������֪��A�Ľṹ��ʽΪ����CH3��2C=C��CH3��2���ʴ�Ϊ����CH3��2C=C��CH3��2��
��2����CH3��2C=C��CH3��2�к���C=C˫����ƽ��ṹ��4������Cԭ�Ӵ���C=C˫����ƽ��ṹ�ڣ�����̼ԭ�Ӷ�����ͬһƽ�棬�ʴ�Ϊ���ǣ�
��3����Ӧ���ǣ�CH3��2C��Cl��C��Cl����CH3��2�����������ƴ���Һ�����������·�����ȥ��Ӧ������CH2=C��CH3��-C��CH3��=CH2���÷�Ӧ�Ļ�ѧ����ʽΪ��CH3��2C��Cl��C��Cl����CH3��2+2NaOH
 CH2=C��CH3��-C��CH3��=CH2+2NaCl+2H2O��
CH2=C��CH3��-C��CH3��=CH2+2NaCl+2H2O��CΪCH2=C��CH3��-C��CH3��=CH2���Ļ�ѧ������2��3-����-1��3-����ϩ��
E2�Ľṹ��ʽ��HOCH2C��CH3��=C��CH3��CH2OH��
��Ӧ���Ǽӳɷ�Ӧ����Ӧ����ȡ����Ӧ��
�ʴ�Ϊ����CH3��2C��Cl��C��Cl����CH3��2+2NaOH
 CH2=C��CH3��-C��CH3��=CH2+2NaCl+2H2O��2��3-����-1��3-����ϩ��HOCH2C��CH3��=C��CH3��CH2OH���ӳɷ�Ӧ��ȡ����Ӧ��
CH2=C��CH3��-C��CH3��=CH2+2NaCl+2H2O��2��3-����-1��3-����ϩ��HOCH2C��CH3��=C��CH3��CH2OH���ӳɷ�Ӧ��ȡ����Ӧ�����������⿼���л����ƶϣ��漰±������ϩ�����ȵ������Լ�����ʽ����⡢ͬ���칹�塢�л���ѧ��Ӧ���ͺͷ���ʽ����д�ȣ���Ŀ�ۺ��Խϴ�ע���ϩ���ļӳɷ�Ӧ���Ѷ��еȣ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
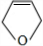

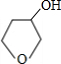 +NaBr
+NaBr