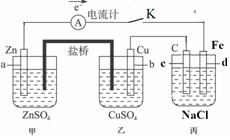
��Ŀ����
��11�֣�����ͼװ�ñպϵ��Kʱ��������A��ָ�뽫����ƫת���Իش�
(1) ������ ���ԭ��ء����ء���������a���缫������ ������c���缫������ ��
(2)����Cu���ĵ缫��Ӧ��______ ������·����0.02mol����ͨ��,�����a�缫�ܽ������Ϊ g��
(3)�պϵ��Kһ��ʱ���,���������ɶ��������һ�ּ������з������ܵĻ�ѧ����ʽ�� ��
��4�������з�Ӧ���нϳ�ʱ����ռ�����״��������2.24L����ʱ��ñ�����Һ����ʵ�ʼ���4.23 g,���м�0.100mol��������������ˮ�е��ܽ⣩����ʵ�ʷų���������ʵ����� mol��
��5�����Ҫ��������Ƭ�϶���һ��Cu�������Ӧ���θĽ� ��
��1������ ���� ����
��2��Cu2++2e��==Cu (2��) 0.65 (3) 2NaCl��2H2O2NaOH��H2����Cl2�� (2��)
��4)0.165 [˵����H2��0.1 Cl2 ��0.05 O2��0.015]
(5)��C�����ɡ�Cu��������NaCl��Һ�����ɡ�CuSO4��Һ������2�֣������Ĵ�Ҳ���֣�
����:����ͨ�������γɱպϻ�·�����пͭԭ��أ��ṩ���ܡ�����װ����Ϊ���ء�
пͭԭ��أ�
������Zn��2e��=Zn2�� ������Cu2����2e��=Cu
�ܷ�ӦΪ��Zn��Cu2��=Zn2����Cu
���ڱ��أ�
���ӵ��������̼��Ϊ������2CI����2e��=Cl2��
���ӵ�ظ���������Ϊ������2H����2e�� = H2��
�ܷ�ӦΪ��2NaCl��2H2O2NaOH��H2����Cl2��
��4��NaOH�����ɵ������������˲���������ʣ��0.1mol����ض���0.05mol��Cl2�ݳ���0.1mol������0.05mol����������֮��Ϊ3.75g��
����Һ���ٵ�����Ϊ4.23g�����Ȼ��ˮ�ĵ�⣬�ݳ���������2H2O2H2����O2��
�ݳ�������������Ϊ4.23-3.75=0.48g���������������ʵ���Ϊ0.015mol��
���߹�0.165mol��
��5�����Ҫ��Ƽ�Ϊ�������Ʋ����Ϊ�������������Һ��������������������ͬ��

 �п������п��Ծ����ϵ�д�
�п������п��Ծ����ϵ�д�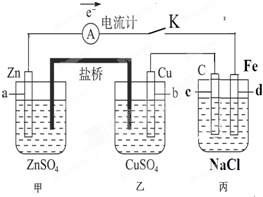 ��ͼװ�ñպϵ��Kʱ��������A��ָ�뽫����ƫת���Իش�
��ͼװ�ñպϵ��Kʱ��������A��ָ�뽫����ƫת���Իش�