��Ŀ����
��12�֣���������"��ָ��ֲ����������������õ������ʵ��ܳơ����������ܡ�����������ԴΣ����һ����Ҫ����Դ���������ʡ���һ�������¿�����ΪCO��H2��ԭ�����������ϳɼ״��Ͷ����ѣ�CH3OCH3),��ط�ӦΪ��
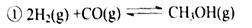

�����ͼʾ�ش����⣺
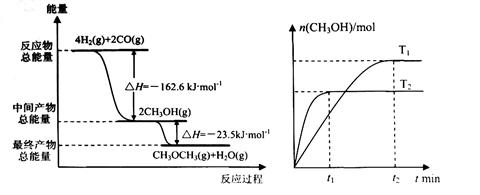
(1) ��H2��CO�ϳɶ����ѵ��Ȼ�ѧ����ʽ�� ��
(2) ��T1��T2�¶��£��������ݻ���ͬ���ܱ������зֱ�ͨ��1molCO��2molH2�ϳɼ״�����T1��T2�¶��¶�Ӧ��Ӧ��ƽ�ⳣ��K1_______K2(ѡ�<������>����=��)��
(3) ��һ�������£���һ���ݻ��ɱ���ܱ������г���4molH2��2molCO��1molCH3OCH3��g����1molH20(g),��һ��ʱ�䷴Ӧ�ڴﵽƽ��״̬����ʱ��û�������ܶ�����ͬ��������ʼʱ��1.6������Ӧ��ʼʱ�����淴Ӧ���ʵĴ�С��ϵΪV(��)_______V(�棩��ѡ� >������< ����=��)��ƽ��ʱ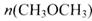 =_________mol��
=_________mol��
(4) ��ͼΪ��ɫ��Դ��������ȼ�ϵ�ء��Ĺ���ԭ��ʾ��ͼ����a��b��Ϊ�����Pt�缫��
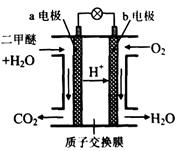
b�缫��_______�����������������
a�缫�ϵĵ缫��ӦΪ___ ____��
��12�֣�(1) 4H2(g)+2CO(g)  CH3OCH3(g)+H2O(g) ��H����186.1 kJ/mol
CH3OCH3(g)+H2O(g) ��H����186.1 kJ/mol
(2) �� (3) �� �� 1.75��4���� CH3OCH3 �C 12e- +3H2O = 2CO2 + 12H+
��������
�����������1������ͼ���֪���÷�Ӧ�ķ�Ӧ�ȡ�H����162.6kJ/mol��23.5 kJ/mol����186.1 kJ/mol�����Ը÷�Ӧ���Ȼ�ѧ����ʽ��4H2(g)+2CO(g)  CH3OCH3(g)+H2O(g) ��H����186.1 kJ/mol��
CH3OCH3(g)+H2O(g) ��H����186.1 kJ/mol��
��2������ͼ���֪T2�������ȴﵽƽ��״̬��˵��T2��T1�����¶�Խ�ߣ��״������ʵ���Խ�٣���˵�������¶ȣ�ƽ�����淴Ӧ�����ƶ���������Ӧ�Ƿ��ȷ�Ӧ������K1��K2��
��3�����ݷ���ʽ��֪���÷�Ӧ�������С�ġ�����������������䣬�������ƽ��ʱ��������ܶ�����ͬ��������ʼʱ��1.6������˵����Ӧ��������Ӧ������еģ���˷�Ӧ��ʼʱ�����淴Ӧ���ʵĴ�С��ϵΪV(��) ��V(�棩�� 4H2(g)+2CO(g)
 CH3OCH3(g)+H2O(g)
CH3OCH3(g)+H2O(g)
��ʼ����mol�� 4 2 1 1
ת������mol�� 4x 2x x x
ƽ������mol��4��4x 2��2x 1��x 1��x
��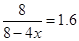
���x��0.75mol
����ƽ��ʱ�����ѵ����ʵ�����1.75mol
��4�������õ����ӣ�������ͨ�룬����b�缫��������a�缫�Ǹ�����������ʧȥ���ӣ����ڴ������ӽ���Ĥ�����Ը����缫��Ӧʽ��CH3OCH3��12e-��3H2O ��2CO2��12H+��
���㣺���鷴Ӧ�ȵļ��㡢ƽ�ⳣ�����жϡ����淴Ӧ���йؼ����Լ��缫���Ƶ��жϺ͵缫��Ӧʽ����д
�����������Ǹ߿��еij������ͣ������ۺ���ǿ���ѶȽϴ�����ע�ػ�����������˫�飬����������ѧ���������⡢��������������Ҳ����������ѧ����Ӧ�����������ѧ����ѧϰЧ�ʡ�

 ������������Ӧ����ϵ�д�
������������Ӧ����ϵ�д� ͬ����չ�Ķ�ϵ�д�
ͬ����չ�Ķ�ϵ�д�