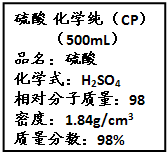
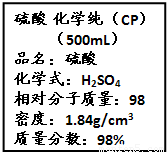
��Ŀ����
��1��NH3��Ħ������Ϊ
��2��������30.0mL��ˮ��70.0mL 2.00mol?L-1��AlCl3��Һ��ϣ����ɵij�������Ϊag��ͨ���������a��ֵ��
17
17
g/mol��0.5mol NH3������Ϊ8.5
8.5
g������״����11.2L��������100mLˮ�У��õ��ܶ�Ϊ0.868g?cm-3�İ�ˮ�����ð�ˮ�����ʵ���Ũ��Ϊ4.00
4.00
mol/L����2��������30.0mL��ˮ��70.0mL 2.00mol?L-1��AlCl3��Һ��ϣ����ɵij�������Ϊag��ͨ���������a��ֵ��
3.12g
3.12g
����������1��Ħ����������Է�����������ֵ��ȣ����m=nM��n=
��c=
���㣻
��2���жϹ�����Ȼ������Al3++3NH3?H2O=Al��OH�� 3��+3NH4+���㣮
| V |
| Vm |
| n |
| V |
��2���жϹ�����Ȼ������Al3++3NH3?H2O=Al��OH�� 3��+3NH4+���㣮
����⣺��1����������Է�������Ϊ17������Ħ������Ϊ17g/mol��0.5mol NH3������Ϊ0.5mol��17g/mol=8.5g��
����״����11.2L��������100mLˮ�У��õ��ܶ�Ϊ0.868g?cm-3�İ�ˮ��n=
=0.5mol����Һ����Ϊ0.5mol��17g/mol+100g=108.5g��
��Һ�����Ϊ
=125mL=0.125L������c=
=4.00mol/L��
�ʴ�Ϊ��17��8.5��4.00��
��2��n��NH3?H2O��=0.03L��4mol/L=0.12mol��
n��AlCl3��=0.07L��2mol/L=0.14mol��
��Al3++3NH3?H2O=Al��OH�� 3��+3NH4+��
1 3 1
��Al3+��������NH3?H2O�������㣬
��Al��OH��3 �����ʵ���0.12mol��
=0.04mol��
Al��OH��3 ������Ϊ0.04mol��78g/mol=3.12g��
�ʴ�Ϊ��3.12g��
����״����11.2L��������100mLˮ�У��õ��ܶ�Ϊ0.868g?cm-3�İ�ˮ��n=
| 11.2L |
| 22.4L/mol |
��Һ�����Ϊ
| 108.5g |
| 0.868g/cm3 |
| 0.5mol |
| 0.125L |
�ʴ�Ϊ��17��8.5��4.00��
��2��n��NH3?H2O��=0.03L��4mol/L=0.12mol��
n��AlCl3��=0.07L��2mol/L=0.14mol��
��Al3++3NH3?H2O=Al��OH�� 3��+3NH4+��
1 3 1
��Al3+��������NH3?H2O�������㣬
��Al��OH��3 �����ʵ���0.12mol��
| 1 |
| 3 |
Al��OH��3 ������Ϊ0.04mol��78g/mol=3.12g��
�ʴ�Ϊ��3.12g��
���������⿼�����ʵ������йؼ��㣬����ѧ�����������Ŀ��飬ע������йؼ��㹫ʽ���������㼴�ɽ����Ŀ�ѶȲ���

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
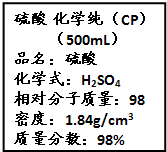 ��Ҫ���������С�⣮
��Ҫ���������С�⣮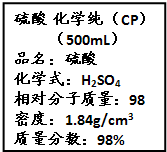 ��Ҫ���������С�⣮
��Ҫ���������С�⣮