��Ŀ����
��H2S��HF��HCl��HI��CH4��NH3��H2O��MgO��SiO2��CO2��SO2��NaCl��NaOH�Ȼ����ﰴ����Ҫ����գ�
��1������ǿ���Լ��������� ������ǿ���Լ���ǿ���� �����������Լ���ǿ���� �����������Լ��������� ����ˮ��Һ��ǿ���Ե��� ����ˮ��Һ�������Ե��� ��
��2���������ӻ�������� ��
��3�����ڷ��Ӿ������������ ������ԭ�Ӿ������������ ��
��4�������������幹�͵Ļ������� ��������ˮ����̬�⻯���� ��������ˮ���������� ��
��1��HF��HCl��HI��H2S��NaOH��NH3 ��2��MgO��NaCl��NaOH��
��3��CO2��SO2��H2O��SiO2 ��4��CH4��CH4��MgO��SiO2
��������
�����������1��F����ǿ�ķǽ�����������������ᣬ��˾���ǿ���Լ���������HF������ǿ���Լ���ǿ���������ᣬ���Ȼ����ˮ��Һ����ķǽ����ԱȽ��������������ǿ������������Լ�����������H2S������������ǿ�����ˮ��ǿ���ԣ���������ˮ����Һ�Լ��ԣ�����ˮ�����
��2���������Ӽ��Ļ����������ӻ��������MgO��NaCl��NaOH�������ӻ����
��3�����Ӽ�ͨ�����Ӽ��������γɵľ����Ƿ��Ӿ��壬�������ڷ��Ӿ������������CO2��SO2��H2O����������������ԭ�Ӿ��塣
��4�������������幹�͵Ļ������Ǽ��飻H2S��HF��HCl��HI��CH4��NH3����������ˮ��������ˮ�ģ���������������ˮ����̬�⻯�H2O��CO2��SO2�ȶ��ǿ�������ˮ�ģ���MgO��SiO2����������ˮ�������
���㣺���鳣������������ʡ��������͡���������й��жϵ�
�����������dz�ʶ��֪ʶ�Ŀ��飬�ѶȲ�����Ҫ�ǿ���ѧ���Ի�������֪ʶ����Ϥ���ճ̶ȣ��Լ��������⡢�������������������ڵ���ѧ����ѧϰ��Ȥ��

 D.NH3�ĽṹʽΪ��
D.NH3�ĽṹʽΪ��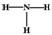
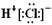 D.NH3�ĽṹʽΪ��
D.NH3�ĽṹʽΪ��