��Ŀ����
����Ŀ�������ƻ���к��е��ۡ������Ǻ����εȣ�ij������ȤС�������һ��ʵ��֤��ijЩ�ɷݵĴ��ڣ�������벢Э������������ʵ�飮
��1����С�Թ�ȡ������ƻ��֭����������Cu(OH)2����Һ�������ȣ�����ש��ɫ�ij�������ƻ���к���_______________��д�ṹ��ʽ)��
��2����������һ�������¿��Եõ���ѧʽΪC2H6O�Ļ�����A��A+CH3COOH������ζ�IJ����A���������Ϊ75%��ˮ��Һ��������_______________��
��3��ƻ���к���ƻ���ᣬ�������Է�������Ϊ134��ȡ0.02molƻ���ᣬʹ����ȫȼ�գ���ȼ�պ�IJ����Ⱥ�ͨ����������ˮCaCl2�ͼ�ʯ�ң����߷ֱ�����1.08g �� 3.52g���������C��Hԭ�ӵĸ�����_______________��ƻ����ķ���ʽ��_______________��
��4����ƻ��������������ʣ���1molƻ���������������Ʒ�Ӧ����1.5mol���壻������Ũ����ͼ��������£�ƻ�����봼���������Ӧ��������ζ�IJ����ƻ������һ�������µķ�������ˮ����ɺ���ˮ�����ӳɷ�Ӧ����1molƻ��������2mol̼�����Ʒ�Ӧ������������Ϣ���Ʋ�ƻ������ܵ�һ�ֽṹ��ʽ��_____________��
���𰸡� ��CH2OH(CHOH)4CHO�� ������ 2��3 C4H6O5 HOOCCH(OH)CH2COOH��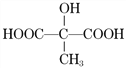
��������(1)��������Cu(OH)2����Һ�������ȣ�����ש��ɫ�ij������������ǣ���ṹ��ʽΪCH2OH(CHOH)4CHO��
(2)C2H6O�Ļ�����A��A+CH3COOH������ζ�IJ��AΪ�Ҵ�����A���������Ϊ75%��ˮ��Һ����������������
(3)ʹ��ˮCaCl2���ؿ�֪ˮ������Ϊ1.08g��n(H2O)= ![]() =0.06 mol��n(H)=0.12 mol��ʹ��ʯ������3.52g����֪������̼����Ϊ3.52g��n(C)=n(CO2)=
=0.06 mol��n(H)=0.12 mol��ʹ��ʯ������3.52g����֪������̼����Ϊ3.52g��n(C)=n(CO2)= ![]() =0.08 mol��1molƻ���Ậ��ԭ��n(H)=6 mol��n(C)=4 mol����n(O)��
=0.08 mol��1molƻ���Ậ��ԭ��n(H)=6 mol��n(C)=4 mol����n(O)�� ![]() =5mol��������C��Hԭ�ӵĸ�����Ϊ0.08mol��0.12mol=2��3����n(C)��n(H)��n(O)=4mol��6mol��5mol=4��6��5������ʽΪC4H6O5��
=5mol��������C��Hԭ�ӵĸ�����Ϊ0.08mol��0.12mol=2��3����n(C)��n(H)��n(O)=4mol��6mol��5mol=4��6��5������ʽΪC4H6O5��
(4)�ɢ�1molƻ���������������Ʒ�Ӧ����1.5mol���壻����������ͼ��������£�ƻ�����봼���������Ӧ��������ζ�IJ����ƻ������һ�������µķ�������ˮ����(���ǻ�״������)�ɺ���ˮ�����ӳɷ�Ӧ����-OH��-COOH����1molƻ��������2mol̼�����Ʒ�Ӧ��˵�����ӽṹ�к���2���Ȼ�������ṹ��ʽΪHOOCCH(OH)CH2COOH��![]() ��
��

 ѧ���쳵��������������������ϵ�д�
ѧ���쳵��������������������ϵ�д�


