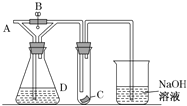
��Ŀ����
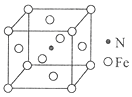
����Ŀ���û�ԭ�����Ʊ���ï�������˽����л��������о�������������ï��������������һ����Ҫ��������ṹ��ͼ��ʾ��
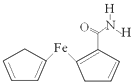
��1����̬Feԭ�Ӽ۲�����Ų�ʽ��_______��
��2����֪��ï���������۵���176�����е���249����������ˮ���������ȷ¡���ͪ���л��ܼ����ݴ˿��ƶ϶�ï������������Ϊ_______���塣
��3����ï����������̼ԭ�ӵ��ӻ���ʽΪ_________��H��C��N��O����Ԫ�صĵ縺���ɴ�С��˳����_________��
��4��̼����Ԫ�ض�Ӧ������⻯��ֱ���CH4��NH3����ͬ������CH4�ķе��NH3�ķе�_______������������������������Ҫԭ����_______��
��5���⡢����������Ԫ�ذ���4:2:3��ԭ�Ӹ������γ����ӻ���������ӻ������У������ӿռ乹����_______��1 mol�����Ӻ�����������ĿΪ_______��
��6�����������γ�һ�ִ��Բ��ϣ��侧����ͼ��ʾ���ô��Բ��ϵĻ�ѧʽΪ_______����֪��������Ϊa nm����þ����ܶȵļ���ʽΪ����_______g/cm3����NA��ʾ�����ӵ�������ֵ����
���𰸡� 3d64s2 ���� sp2��sp3 O>N>C>H �� �����Ӽ������� ���������� 3NA��1.806��1024 Fe4N 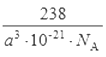
��������������Ҫ�������ʽṹ��
��1����̬Feԭ�Ӽ۲�����Ų�ʽ��3d64s2��
��2����ï���������۵㡢�е�ϵͣ�������ˮ���������ȷ¡���ͪ���л��ܼ����ݴ˿��ƶ϶�ï������������Ϊ���Ӿ��塣
��3����ï����������˫��̼ԭ�ӵ��ӻ���ʽΪsp2������̼ԭ�ӵ��ӻ���ʽΪsp3���ǽ����ԣ�H<C<N<O����������Ԫ�صĵ縺���ɴ�С��˳����O>N>C>H��
��4��̼����Ԫ�ض�Ӧ������⻯��ֱ���CH4��NH3����ͬ������CH4�ķе��NH3�ķе�ͣ���Ҫԭ���ǰ����Ӽ���������
��5���⡢����������Ԫ�ذ���4:2:3��ԭ�Ӹ������γ����ӻ�����NH4NO3�������ӻ������У������ӿռ乹�������������Σ�1 mol�����Ӻ�����������ĿΪ3NA��1.806��1024��
��6����������1����ԭ�Ӻ�4����ԭ�ӣ����ǵ����ԭ������֮��Ϊ238���ô��Բ��ϵĻ�ѧʽΪFe4N���þ������Ϊa3 ��10-21cm3������Ϊ238g/NA����þ����ܶȵļ���ʽΪ��=![]() g/cm3��
g/cm3��

 53���ò�ϵ�д�
53���ò�ϵ�д�����Ŀ����֪����������(NiC2O4��2H2O)������ˮ����ҵ�ϴӷ�������(��Ҫ�ɷ�ΪNi������һ������Al2O3��FeO��SiO2��CaO��)�Ʊ������������������ͼ��ʾ��

��֪������ؽ������������������������pH���������ݣ�
�������� | Fe3�� | Fe2�� | Al3�� | Ni2�� |
��ʼ������pH | 1.1 | 5.8 | 3.0 | 6.8 |
��ȫ������pH | 3.2 | 8.8 | 5.0 | 9.5 |
��Ksp(CaF2)��1.46��10��10��
����ij����Ũ��С��1.0��10��5 mol��L��1ʱ����Ϊ��ȫ������
��ش��������⣺
��1����д��һ�����������������ʵĴ�ʩ��________________��
��2���Լ�a��һ����ɫ��������д����������ʱ��Ӧ�����ӷ���ʽ��_______________��
��3������pH��ʱpH�ĵ��ط�ΧΪ_____________�����û�ѧ��Ӧԭ�������֪ʶ���������������ɣ�______________________________________________________��
��4��д����������ʱ������Ӧ�����ӷ���ʽ��__________________________________��֤��Ni2���Ѿ�������ȫ��ʵ�鲽�輰������___________________________________����Ca2��������ȫʱ����Һ��c(F��)>____________mol��L��1(д������ʽ����)��
��5������a��������_________________________________________________________��
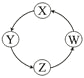
����Ŀ�����и��������У��������ͼʾ������ͨ����������һ��ת����ȫ�������
��� | X | Y | Z | W |
|
�� | Cu | CuSO4 | Cu(OH)2 | CuO | |
�� | Na | NaOH | Na2CO3 | NaCl | |
�� | Cl2 | Ca(ClO)2 | HClO | HCl | |
�� | Fe | FeCl3 | FeCl2 | Fe(OH)2 |
A. �٢ڢ� B. �٢ۢ� C. �ڢ� D. �٢�