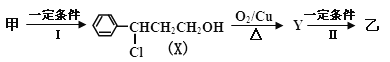ЬтФПФкШн
ЁОЬтФПЁПЯТЭМЪЧвЛИіЕчЛЏбЇЙ§ГЬЕФЪОвтЭМЃЌЛиД№ЯТСаЮЪЬтЃК

ЃЈ1ЃЉМзГиНЋ______ФмзЊЛЏЮЊ______ФмЃЌввзАжУжаЕчМЋAЪЧ____МЋЁЃ
ЃЈ2ЃЉМззАжУжаЭЈШыCH4ЕФЕчМЋЗДгІЪНЮЊ__________________________________ЃЌввзАжУжаЕчМЋB(Ag)ЕФЕчМЋЗДгІЪНЮЊ________________________________ЁЃ
ЃЈ3ЃЉвЛЖЮЪБМфЃЌЕББћГижаВњЩњ112 mL(БъзМзДПі)ЦјЬхЪБЃЌОљдШНСАшБћГиЃЌЫљЕУШмвКдк25 ЁцЪБЕФpHЃН________(вбжЊЃКNaClШмвКзуСПЃЌЕчНтКѓШмвКЬхЛ§ЮЊ500 mL)ЁЃШєвЊЪЙБћГиЛжИДЕчНтЧАЕФзДЬЌЃЌгІЯђБћГижаЭЈШы________(аДЛЏбЇЪН)ЁЃ
ЁОД№АИЁП ЛЏбЇ Еч бє CH4ЃЋ10OHЃЃ8eЃ=CO32- ЃЋ7H2O AgЃЋЃЋeЃ=Ag 12 HCl
ЁОНтЮіЁПЃЈ1ЃЉМзГижавЛМЋЭЈШыМзЭщЃЌСэвЛМЋЭЈШыбѕЦјЃЌЫљвдМзГиЮЊМзЭщаЮГЩЕФШМСЯЕчГиЃЌЪЧАбЛЏбЇФмБфЮЊЕчФмЕФзАжУЃЛЭЈШыМзЭщЕФвЛМЋЮЊИКМЋЃЌЭЈШыбѕЦјЕФвЛМЋЮЊе§МЋЃЌAгые§МЋЯрСЌЃЌЫљвдAЮЊбєМЋЃЛе§ШЗД№АИЃКЛЏбЇ ЃЛЕчЃЛ бєЃЛ
(2)![]() дкИКМЋЩЯЪЇЕчзгЃЌМюадЬѕМўЯТЩњГЩЬМЫсИљЃЌЫљвдМззАжУжаЭЈШы
дкИКМЋЩЯЪЇЕчзгЃЌМюадЬѕМўЯТЩњГЩЬМЫсИљЃЌЫљвдМззАжУжаЭЈШы![]() ЕФЕчМЋЗДгІЪНЮЊЃКCH4ЃЋ10OHЃЃ8eЃ=CO32- ЃЋ7H2O ЃЛввзАжУжа
ЕФЕчМЋЗДгІЪНЮЊЃКCH4ЃЋ10OHЃЃ8eЃ=CO32- ЃЋ7H2O ЃЛввзАжУжа![]() ЕФЕчМЋЮЊвѕМЋЃЌШмвКжаЕФвјРызгЕУЕчзгЃЌЦфЕчМЋЗДгІЪНЮЊ: AgЃЋЃЋeЃ=Ag ЃЛе§ШЗД№АИЃКCH4ЃЋ10OHЃЃ8eЃ=CO32- ЃЋ7H2O ЃЛ AgЃЋЃЋeЃ=Ag ЁЃ
ЕФЕчМЋЮЊвѕМЋЃЌШмвКжаЕФвјРызгЕУЕчзгЃЌЦфЕчМЋЗДгІЪНЮЊ: AgЃЋЃЋeЃ=Ag ЃЛе§ШЗД№АИЃКCH4ЃЋ10OHЃЃ8eЃ=CO32- ЃЋ7H2O ЃЛ AgЃЋЃЋeЃ=Ag ЁЃ
ЃЈ3ЃЉгУСНИіЖшадЕчМЋЕчНтТШЛЏФЦШмвКЃЌзмЗДгІЮЊ2NaCl+2H2O=2NaOH+Cl2Ёќ+H2Ёќ, ВњЩњ112 mL(БъзМзДПі)ЦјЬхЪБЃЌЦјЬхЕФСПЮЊ0.005molЃЌВњЩњЧтЦјКЭТШЦјЗжБ№ЮЊ0.0025molЃЌИљОнЗНГЬЪНПЩжЊЃЌЩњГЩNaOHЕФСПЮЊ0.005molЃЌc(OH-)=0.005/0.5=0.01mol/LЃЌc(H+)=10-12 mol/LЃЌpHЃН12ЃЛвђЮЊЕчНтЩњГЩ![]() КЭ
КЭ![]() ДгШмвКжавнГіЃЌЫљвдгІИУМгЖўепЕФЛЏКЯЮяЃЌМДМгШыHClЃЛе§ШЗД№АИЃК12ЃЛ HClЁЃ
ДгШмвКжавнГіЃЌЫљвдгІИУМгЖўепЕФЛЏКЯЮяЃЌМДМгШыHClЃЛе§ШЗД№АИЃК12ЃЛ HClЁЃ

 дФЖСПьГЕЯЕСаД№АИ
дФЖСПьГЕЯЕСаД№АИЁОЬтФПЁПЃЈ1ЃЉЯТБэПеИёжаЕФЬўЗжзгЪНЮЊ_______ЃЌЦфга_______жжЭЌЗжвьЙЙЬхЁЃ
1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
CH4 | C2H4 | C3H8 | C4H8 | C6H12 | C7H16 | C8H16 |
ЃЈ2ЃЉЛЏКЯЮя(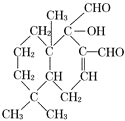 )ЪЧвЛжжаТаЭЩБГцМСЃЌЦфКЌбѕЙйФмЭХЕФУћГЦЮЊ_____________________ЁЃ
)ЪЧвЛжжаТаЭЩБГцМСЃЌЦфКЌбѕЙйФмЭХЕФУћГЦЮЊ_____________________ЁЃ
ЃЈ3ЃЉCH2=CH-![]() -CЁдC-CH3жаЃЌзюЖрга_______ИідзгЙВУцЁЃ
-CЁдC-CH3жаЃЌзюЖрга_______ИідзгЙВУцЁЃ
ЃЈ4ЃЉЂйИљОнУћГЦЪщаДНсЙЙМђЪНЃК2-МзЛљ-2,4-вбЖўЯЉ_________________________________
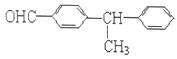
ЃЈ5ЃЉгаЛњЮя![]() ЕФЯЕЭГУќУћЪЧ___________________________________ЃЌНЋЦфдкДпЛЏМСДцдкЯТЭъШЋЧтЛЏЃЌЫљЕУЭщЬўЕФЯЕЭГУќУћЪЧ_________________________________
ЕФЯЕЭГУќУћЪЧ___________________________________ЃЌНЋЦфдкДпЛЏМСДцдкЯТЭъШЋЧтЛЏЃЌЫљЕУЭщЬўЕФЯЕЭГУќУћЪЧ_________________________________
ЃЈ6ЃЉ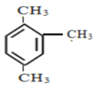 ЕФЯЕЭГУќУћЪЧ______________________________
ЕФЯЕЭГУќУћЪЧ______________________________