��Ŀ����
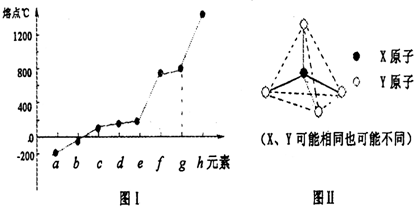
��11�֣��ɶ�����Ԫ����ɵ�10������A��J������ͼ��ʾ��ת����ϵ����֪A��BΪͬ��������Ԫ�صĵ��ʣ������Ϊ�����ͨ��״����AΪ���壬B��DΪ���壬FΪҺ�壻A��G��Ũ��Һ����ʱ��Ӧ����D��F��J�ڹ���ʱ��I���ɡ��Իش�
(1)д���������ʵĻ�ѧʽ��B_________��E__________��F�Ľṹʽ__________��
(2)��Ӧ�ٵ����ӷ���ʽΪ______________________________��
(3)��������G���ȵ�Ũ��Һ��Ӧ���������� ��
����B��Ӧ�Ļ�ѧ����ʽ ��
(4)C��һ����Ҫ�Ļ�����Ʒ��Ϊ�ӷ���ӣ�Һ�ɫҺ�塣����Ӧ�������ɵ�G��A��I�����ʵ���֮��1�U2�U6����C�ĵ���ʽΪ ����Ӧ�ڵĻ�ѧ����ʽΪ__ ��
(1)Cl2��SO3��H��O��H(ÿ��1�֣���3��)
(2)HClO��SO2��H2O=3H����SO42����Cl��(2��) (3)SO2��H2(2��) (1��) (4)
(1��) (4) (2��)
(2��)
3SCl2��4H2O=H2SO4��2S����6HCl(2��)
����

��ϰ��ϵ�д�
�����Ŀ


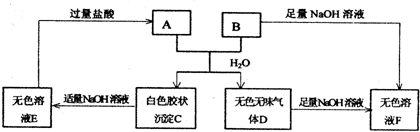
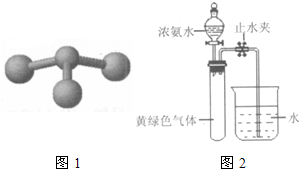 ��2010?������һģ���ס��ҡ����������ɶ�����Ԫ����ɵ����ʣ�����֮���������ת����ϵ����+�ҡ���+��
��2010?������һģ���ס��ҡ����������ɶ�����Ԫ����ɵ����ʣ�����֮���������ת����ϵ����+�ҡ���+��
 �ס����������ɶ�����Ԫ����ɵ����ʣ�����֮���������ת����ϵ��
�ס����������ɶ�����Ԫ����ɵ����ʣ�����֮���������ת����ϵ��