��Ŀ����
��һ������ˮ��Һ��ֻ���ܺ������������е������֣�K+��NH4+��Cl-��Mg2+��CO32-��SO42-����ȡ����100.00ml��Һ��������ʵ�飺
�ٵ�һ�ݼ���AgNO3��Һ�г���������
�ڵڶ��ݼ�����NaOH��Һ���Ⱥ��ռ�������0.896L����״̬�£���
�۵����ݼ�����BaCl2��Һ�ø������6.27g������������ϴ�ӡ������������Ϊ2.33g������ʵ��ش��������⣺
��1���ж�K+��Cl-�Ƿ���ڣ�K+______��Cl-______����������ţ�
A��һ�����ڡ������� B�����ܴ��ڡ��������� C��һ��������
��2��ԭ��Һ�У��϶����ڵĸ����ӵ����ʵ���Ũ�ȷֱ�Ϊ���٣�
�⣺���������Ϣ�������ٵ�һ�ݼ���AgNO3��Һ�г���������˵������Cl-��CO32-��SO42-��
�ڵڶ��ݼ�����NaOH��Һ���Ⱥ��ռ�������0.896L����״̬�£���֤��һ������NH4+�����ɵ�����Ϊ�������ʵ���=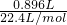 =0.04mol��
=0.04mol��
�۵����ݼ�����BaCl2��Һ�ø������6.27g������������ϴ�ӡ������������Ϊ2.33g��֤��ԭ��Һ��һ������CO32-��SO42-�����ɳ���BaSO4���ʵ���=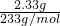 =0.01mol�����ɵij���BaCO3���ʵ���=
=0.01mol�����ɵij���BaCO3���ʵ���= =0.02mol���������ӹ����֪��Һ��һ������Mg2+��K+��Cl-����ȷ����
=0.02mol���������ӹ����֪��Һ��һ������Mg2+��K+��Cl-����ȷ����
��1��������Һ�е���غ���㣺һ������CO32-��SO42-��NH4+�������=0.04mol�������=0.02mol��2+0.01mol��2=0.06mol������һ��������һ��������K+�������ӿ��ܺ��У�
�ʴ�Ϊ��A��B��
��2��ԭ��Һ�У��϶����ڵĸ����ӵ����ʵ���Ũ�ȷֱ�Ϊ��C��CO32-��=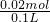 =0.2mol/L��C��SO42-��=
=0.2mol/L��C��SO42-��= =0.1mol/L��C��NH4+��=
=0.1mol/L��C��NH4+��= =0.4mol/L��������Һ�е���غ�õ���C��K+��+C��NH4+��=2C��SO42-��+2C��CO32-����C��K+��=0.2mol/L������Һ�к��������ӣ���C��K+����0.2mol/L��
=0.4mol/L��������Һ�е���غ�õ���C��K+��+C��NH4+��=2C��SO42-��+2C��CO32-����C��K+��=0.2mol/L������Һ�к��������ӣ���C��K+����0.2mol/L��
�ʴ�Ϊ����C��CO32-��=0.2mol/L��C��SO42-��=0.1mol/L��C��NH4+��=0.4mol/L��C��K+����0.2mol/L��
�������ٵ�һ�ݼ���AgNO3��Һ�г���������˵������Cl-��CO32-��SO42-��
�ڵڶ��ݼ�����NaOH��Һ���Ⱥ��ռ�������0.896L����״̬�£���֤��һ������NH4+�����ɵ�����Ϊ�������ʵ���=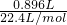 =0.04mol��
=0.04mol��
�۵����ݼ�����BaCl2��Һ�ø������6.27g������������ϴ�ӡ������������Ϊ2.33g��֤��ԭ��Һ��һ������CO32-��SO42-�����ɳ���BaSO4���ʵ���=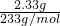 =0.01mol�����ɵij���BaCO3���ʵ���=
=0.01mol�����ɵij���BaCO3���ʵ���= =0.02mol���������ӹ����֪��Һ��һ������Mg2+��K+��Cl-����ȷ����
=0.02mol���������ӹ����֪��Һ��һ������Mg2+��K+��Cl-����ȷ����
���������⿼���˳������Ӽ��鷽����Ӧ�ã��������ʵ����ļ��㣬��Һ�е���غ��Ӧ�ã����ʺͷ�Ӧ�������жϵ����ݣ���Һ���������ж����Ӵ��ڵĹؼ���
�ڵڶ��ݼ�����NaOH��Һ���Ⱥ��ռ�������0.896L����״̬�£���֤��һ������NH4+�����ɵ�����Ϊ�������ʵ���=
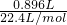 =0.04mol��
=0.04mol���۵����ݼ�����BaCl2��Һ�ø������6.27g������������ϴ�ӡ������������Ϊ2.33g��֤��ԭ��Һ��һ������CO32-��SO42-�����ɳ���BaSO4���ʵ���=
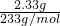 =0.01mol�����ɵij���BaCO3���ʵ���=
=0.01mol�����ɵij���BaCO3���ʵ���= =0.02mol���������ӹ����֪��Һ��һ������Mg2+��K+��Cl-����ȷ����
=0.02mol���������ӹ����֪��Һ��һ������Mg2+��K+��Cl-����ȷ������1��������Һ�е���غ���㣺һ������CO32-��SO42-��NH4+�������=0.04mol�������=0.02mol��2+0.01mol��2=0.06mol������һ��������һ��������K+�������ӿ��ܺ��У�
�ʴ�Ϊ��A��B��
��2��ԭ��Һ�У��϶����ڵĸ����ӵ����ʵ���Ũ�ȷֱ�Ϊ��C��CO32-��=
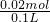 =0.2mol/L��C��SO42-��=
=0.2mol/L��C��SO42-��= =0.1mol/L��C��NH4+��=
=0.1mol/L��C��NH4+��= =0.4mol/L��������Һ�е���غ�õ���C��K+��+C��NH4+��=2C��SO42-��+2C��CO32-����C��K+��=0.2mol/L������Һ�к��������ӣ���C��K+����0.2mol/L��
=0.4mol/L��������Һ�е���غ�õ���C��K+��+C��NH4+��=2C��SO42-��+2C��CO32-����C��K+��=0.2mol/L������Һ�к��������ӣ���C��K+����0.2mol/L���ʴ�Ϊ����C��CO32-��=0.2mol/L��C��SO42-��=0.1mol/L��C��NH4+��=0.4mol/L��C��K+����0.2mol/L��
�������ٵ�һ�ݼ���AgNO3��Һ�г���������˵������Cl-��CO32-��SO42-��
�ڵڶ��ݼ�����NaOH��Һ���Ⱥ��ռ�������0.896L����״̬�£���֤��һ������NH4+�����ɵ�����Ϊ�������ʵ���=
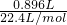 =0.04mol��
=0.04mol���۵����ݼ�����BaCl2��Һ�ø������6.27g������������ϴ�ӡ������������Ϊ2.33g��֤��ԭ��Һ��һ������CO32-��SO42-�����ɳ���BaSO4���ʵ���=
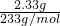 =0.01mol�����ɵij���BaCO3���ʵ���=
=0.01mol�����ɵij���BaCO3���ʵ���= =0.02mol���������ӹ����֪��Һ��һ������Mg2+��K+��Cl-����ȷ����
=0.02mol���������ӹ����֪��Һ��һ������Mg2+��K+��Cl-����ȷ�������������⿼���˳������Ӽ��鷽����Ӧ�ã��������ʵ����ļ��㣬��Һ�е���غ��Ӧ�ã����ʺͷ�Ӧ�������жϵ����ݣ���Һ���������ж����Ӵ��ڵĹؼ���

��ϰ��ϵ�д�
�����Ŀ
����һ������ˮ��Һ��ֻ���ܺ������������е������֣�Na+��NH4+��Ba2+��Cl-��CO32-��SO42-����ȡ����200mL��Һ��������ʵ�飬����ʵ�������Ʋ���ȷ���ǣ�������
�ٵ�һ�ݼ�����NaOH��Һ�����ȣ��ռ�������1.36g��
�ڵڶ��ݼ�����BaCl2��Һ�ø������12.54g������������ϴ�ӡ������������Ϊ4.66g��
�ٵ�һ�ݼ�����NaOH��Һ�����ȣ��ռ�������1.36g��
�ڵڶ��ݼ�����BaCl2��Һ�ø������12.54g������������ϴ�ӡ������������Ϊ4.66g��
| A��һ��������Ba2+��NH4+���ܴ��� | B��CO32-һ�������� | C��Na+һ������ | D��һ��������Cl- |