��Ŀ����
��1�����ױ������ϵ�һ�����������ͬ���칹�壬��Щһ��������������Ķ�Ӧ���ױ����۵�ֱ�Ϊ��
| һ������ױ� | 234�� | 206�� | 213.8�� | 204�� | 214.5�� | 205 |
| ��Ӧ�Ķ��ױ� | -13�� | -54�� | -27�� | -54�� | -27�� | -54�� |
��2����ȫȼ���������ٱ��飨C3H8�����ڶ�ϩ��C4H8��������ϩ��C2H4�����ܼ��飨C6H14���������ʵ�������������������������С��Ϊ ����д��ţ���������ʱ�������������Ϊ ����д��ţ���
��3��0.2 molij��A����������ȫȼ�պ�����CO2��H2O��1.2 mol���Իش�
�� ����A����ʹ��ˮ��ɫ������һ��������������������ȡ����Ӧ����һ��ȡ����ֻ��һ�֣�����A�Ľṹ��ʽΪ__________________��
�� ����A��ʹ��ˮ��ɫ���ڴ��������£���H2�ӳɷ�Ӧ������2,2-�������飬����A��������________________��
��4����ɷ���CnH2n��2��ij���������ӽṹ��û��֧�������������ȫȼ��ʱ������O2�������ͬ״���¸������������8.5�����ɴ˷����ش�
��������Ϊ��״��ϩ��������������ʵ�����Br2�ӳɺ�ֻ�ܵõ���һ���������Ľṹ��ʽΪ______________________________________
��������ֻ��������ʵ�����Br2�����ӳɷ�Ӧ������ṹ��ʽΪ______________(����һ��)��
��14�֣�
��1��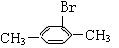 ��2���� ��
��2���� ��
��3����  �� 3��3-����-1-��ϩ
�� 3��3-����-1-��ϩ
��4���� CH2==CH��CH2��CH2��CH==CH2 �� 
���������������1�����л����ͬ���칹���У�֧��Խ�࣬�۵�Խ�ߣ����۵�Ϊ234����ӵĽṹ��ʽΪ ��
��
��2����д��ȼ��ͨʽ��CXHY����̬��+(X+Y/4)O2 XCO2+Y/2H2O����̬����(X+Y/4)ԽС��������ԽС���������ʵ�������������������������С��Ϊ�ۣ�����1molO2ʱ����Ҫ12gC��4gH��������ͬ������������ȫȼ�գ����к�C%Խ�ߣ�����O2Խ�٣���������ʱ������������Ϊ�١�
XCO2+Y/2H2O����̬����(X+Y/4)ԽС��������ԽС���������ʵ�������������������������С��Ϊ�ۣ�����1molO2ʱ����Ҫ12gC��4gH��������ͬ������������ȫȼ�գ����к�C%Խ�ߣ�����O2Խ�٣���������ʱ������������Ϊ�١�
��3��ij��A 0.2mol ����������ȫȼ�պ�����CO2��H2O��1.2mol��������к���N��C��=6��n��H��=12������ʽΪC6H12��
��C6H12ֻ��1�������Ͷȣ�����A����ʹ��ˮ��ɫ������Ϊ�����������У�������������ȡ����Ӧ����һ�ȴ���ֻ��һ�ֵ��ǻ����飬�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
����A��ʹ��ˮ��ɫ���ڴ��������£���H2�ӳɣ���ӳɲ��ᆳ�ⶨ�����к���4������˵�����к���C=C�����У����� 4�������� 3�֣���̼�ܽṹΪ���٢ڢ۴��ɷֱ�ͬʱ����˫������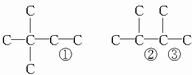 ����A�����еĽṹ��ʽΪ��CH3��3C-CH=CCH2��CH3-C��CH3��=C��CH3��-CH3��CH3CH��CH3��-C��CH3��=CH2�ȣ�
����A�����еĽṹ��ʽΪ��CH3��3C-CH=CCH2��CH3-C��CH3��=C��CH3��-CH3��CH3CH��CH3��-C��CH3��=CH2�ȣ�
�ʴ�Ϊ����CH3��3C-CH=CCH2��CH3-C��CH3��=C��CH3��-CH3��CH3CH��CH3��-C��CH3��=CH2����дһ�֣���
��4����д��ȼ��ͨʽ��CnH2n��2����̬��+(3n-1)/2O2 nCO2+(n-1)H2O����̬����������O2�������ͬ״���¸������������8.5������(3n-1)/2=8.5�����n=6����A��C6H10��
nCO2+(n-1)H2O����̬����������O2�������ͬ״���¸������������8.5������(3n-1)/2=8.5�����n=6����A��C6H10��
��������Ϊ��״��ϩ�������ӽṹ��û��֧���������������ʵ�����Br2�ӳɺ�ֻ�ܵõ���һ���Ҳ���Ǻ���2��̼̼˫�����ҳ���Գ�λ�ã����ṹ��ʽΪCH2==CH��CH2��CH2��CH==CH2��
�ڸ���ֻ��������ʵ�����Br2�����ӳɷ�Ӧ��Ҳ����˵����1��̼̼˫������ṹ��ʽΪ �ȡ�
�ȡ�
���㣺�л���Ľṹ������
���������⿼���л���Ľṹ�����ʣ���Ŀ�Ѷ��еȣ����������ͬ���칹����жϣ�ע��������ʵ������жϿ��ܾ��еĽṹ��

 �ִʾ��ƪϵ�д�
�ִʾ��ƪϵ�д���1�����ױ������ϵ�һ�����������ͬ���칹�壬��Щһ��������������Ķ�Ӧ���ױ����۵�ֱ�Ϊ��
|
һ������ױ� |
234�� |
206�� |
213.8�� |
204�� |
214.5�� |
205 |
|
��Ӧ�Ķ��ױ� |
-13�� |
-54�� |
-27�� |
-54�� |
-27�� |
-54�� |
�۵�Ϊ234����ӵĽṹ��ʽΪ____________
��2����ȫȼ���������ٱ��飨C3H8�����ڶ�ϩ��C4H8��������ϩ��C2H4�����ܼ��飨C6H14���������ʵ�������������������������С��Ϊ ����д��ţ���������ʱ�������������Ϊ ����д��ţ���
��3��0.2 molij��A����������ȫȼ�պ�����CO2��H2O��1.2 mol���Իش�
�� ����A����ʹ��ˮ��ɫ������һ��������������������ȡ����Ӧ����һ��ȡ����ֻ��һ�֣�����A�Ľṹ��ʽΪ__________________��
�� ����A��ʹ��ˮ��ɫ���ڴ��������£���H2�ӳɷ�Ӧ������2,2-�������飬����A��������________________��
��4����ɷ���CnH2n��2��ij���������ӽṹ��û��֧�������������ȫȼ��ʱ������O2�������ͬ״���¸������������8.5�����ɴ˷����ش�
��������Ϊ��״��ϩ��������������ʵ�����Br2�ӳɺ�ֻ�ܵõ���һ���������Ľṹ��ʽΪ______________________________________
��������ֻ��������ʵ�����Br2�����ӳɷ�Ӧ������ṹ��ʽΪ______________(����һ��)��
(1) ���ױ������ϵ�һ�����������ͬ���칹�壬�û�ԭ���Ƶ����ֶ��ױ������ǵ��۵�ֱ����±���
|
��������ױ����۵� |
234�� |
206�� |
213.8�� |
204�� |
214.5�� |
205 |
|
��Ӧ��ԭ���ױ����۵� |
-13�� |
-54�� |
-27�� |
-54�� |
-27�� |
-54�� |
�۵�Ϊ234����ӵĽṹ��ʽΪ____________
�۵�Ϊ-54��ķ��ӵĽṹ��ʽΪ___________��
��2�������������ٱ��飨C3H8�����ڶ�ϩ��C4H8��������ϩ��C2H4�����ܼ��飨C6H14�������ʵ�������������������������С��Ϊ ��������ʱ�������������Ϊ ��
(3) 0.2 molij��A����������ȫȼ�պ�����CO2��H2O��1.2 mol���Իش�
A ����A����ʹ��ˮ��ɫ������һ��������������������ȡ����Ӧ����һ��ȡ����ֻ��һ�֣�����A�Ľṹ��ʽΪ__________________��
B ����A��ʹ��ˮ��ɫ���ڴ��������£���H2�ӳɷ�Ӧ������2,2-�������飬����A��������________________���ṹ��ʽ��____________________��
C ����A������̼ԭ������ʹ��ˮ��ɫ��A��ͬϵ����________��ͬ���칹��