��Ŀ����
��1�������ӵ�����ΪNAmol-1������ij���壬����Ħ��������M g?mol-1����t��1.01×105Paʱ������Ħ�����ΪVm L?mol-1��ȡt��1.01×105Paʱ������m g���ش��������⣺�ٸ�����һ�����ӵ�����______g��
��mg������������������Ŀ______����
��t��1.01×105Paʱ��m g����������______ L��
��2��������O2 ��O3�����ǵ����ʵ���֮��Ϊ______����������ԭ����֮��Ϊ______�����ǵ����֮�ȣ���״����Ϊ______���ܶ�֮�ȣ���״����______��
��3����Ԫ����11H��21H��31H���ֺ��أ���Ԫ��Ҳ��168O��178O��188O���ֺ��أ�������֮���ϳ�ˮ���ӣ�������______�ֲ�ͬ��ˮ���ӣ���������������ͬ����______�֣�
���𰸡���������1����2������n=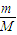 =
=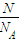 =
=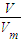 ���������������
���������������
��3����������ϵĽǶȷ�����
����⣺��1���ٺ���1mol�������ʱ�����Ӹ���ΪNA��������ΪMg���������һ�����ӵ�����Ϊ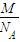 g��
g��
�ʴ�Ϊ��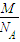 ��
��
��n=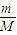 mol��N=
mol��N=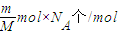 =
=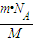 �����ʴ�Ϊ��
�����ʴ�Ϊ��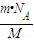 ��
��
��t��1.01×105Paʱ��mg����������Ϊ��V=nVm=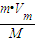 L���ʴ�Ϊ��
L���ʴ�Ϊ��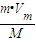 ��
��
��2��M��O2��=32g/mol��M��O3��=48g/mol��
����n=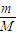 =
=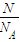 =
=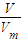 ��֪��������O2��O3�����ǵ����ʵ���֮��Ϊ��
��֪��������O2��O3�����ǵ����ʵ���֮��Ϊ�� =3��2��
=3��2��
���ڶ�������ԭ����ɣ����������ʱ�����е���ԭ�Ӹ�����ȣ�����������ԭ����֮��Ϊ1��1��
���ǵ����֮�������ʵ��������ȣ������֮��Ϊ3��2��
���=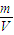 =
=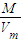 �����ܶ���Ħ��������֮�ȣ����ܶ�֮�ȣ���״����Ϊ��32��48=2��3��
�����ܶ���Ħ��������֮�ȣ����ܶ�֮�ȣ���״����Ϊ��32��48=2��3��
�ʴ�Ϊ��3��2��1��1�� 3��2��2��3��
��3��16O��������H����6��ˮ���ӣ�1-1-16��2-2-16��3-3-16��1-2-16��1-3-16��2-3-16��
������ԭ�ӿ�����18��ˮ���ӣ�
18��ˮ�����У�ʽ����С����18������24����˹���7�ֲ�ͬ��ʽ��
�ʴ�Ϊ��18��7��
���������⿼�����ʵ����ļ��㣬��Ŀ�Ѷ��еȣ�ע�⣨3��Ϊ�״��㣬��������ϵĽǶȷ����ɽ��
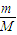 =
=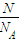 =
=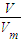 ���������������
�����������������3����������ϵĽǶȷ�����
����⣺��1���ٺ���1mol�������ʱ�����Ӹ���ΪNA��������ΪMg���������һ�����ӵ�����Ϊ
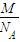 g��
g���ʴ�Ϊ��
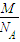 ��
����n=
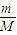 mol��N=
mol��N=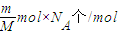 =
=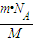 �����ʴ�Ϊ��
�����ʴ�Ϊ��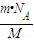 ��
����t��1.01×105Paʱ��mg����������Ϊ��V=nVm=
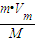 L���ʴ�Ϊ��
L���ʴ�Ϊ��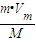 ��
����2��M��O2��=32g/mol��M��O3��=48g/mol��
����n=
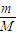 =
=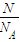 =
=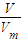 ��֪��������O2��O3�����ǵ����ʵ���֮��Ϊ��
��֪��������O2��O3�����ǵ����ʵ���֮��Ϊ�� =3��2��
=3��2�����ڶ�������ԭ����ɣ����������ʱ�����е���ԭ�Ӹ�����ȣ�����������ԭ����֮��Ϊ1��1��
���ǵ����֮�������ʵ��������ȣ������֮��Ϊ3��2��
���=
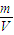 =
=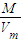 �����ܶ���Ħ��������֮�ȣ����ܶ�֮�ȣ���״����Ϊ��32��48=2��3��
�����ܶ���Ħ��������֮�ȣ����ܶ�֮�ȣ���״����Ϊ��32��48=2��3���ʴ�Ϊ��3��2��1��1�� 3��2��2��3��
��3��16O��������H����6��ˮ���ӣ�1-1-16��2-2-16��3-3-16��1-2-16��1-3-16��2-3-16��
������ԭ�ӿ�����18��ˮ���ӣ�
18��ˮ�����У�ʽ����С����18������24����˹���7�ֲ�ͬ��ʽ��
�ʴ�Ϊ��18��7��
���������⿼�����ʵ����ļ��㣬��Ŀ�Ѷ��еȣ�ע�⣨3��Ϊ�״��㣬��������ϵĽǶȷ����ɽ��

��ϰ��ϵ�д�
�����Ŀ
�ڱ�״��ʱ��m��ij����X�����ΪV������Ħ������ΪM g?mol-1�������ӵ�������ֵΪNA���������йظ������˵������ȷ���ǣ�������
| A��M/NA��ʾ�����嵥�����ӵ����� | B��VM/m��ʾ����������ʵ���Ũ�� | C��M/22.4��ʾ��״���¸�������ܶ� | D��mNA/M��ʾ������ķ����� |
��ijԭ�ӵ�Ħ��������M g?mol-1�������ӵ�������ֵΪ6.02��1023����һ����ԭ�ӵ���ʵ�����ǣ�������
| A��M g | ||
B��
| ||
C��
| ||
D��
|