��Ŀ����
����ѧ--ѡ���л���ѧ������
����ʽΪC12H14O2��F�л���㷺�����㾫�ĵ������
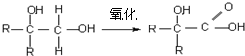
��֪����R-CH2X+NaOH
R-CH2OH+NaCl ��X����±��ԭ�ӣ�
��
Ϊ�˺ϳɸ��ijʵ���ҵĿƼ���Ա��������кϳ�·�ߣ�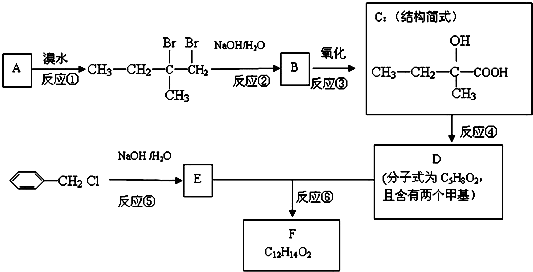
�Իش��������⣺
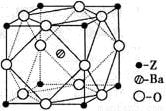
��1��A�����ں˴Ź����������ܳ���
 ��
��
��2�������ϳ�·��������ȡ����Ӧ����
��3����Ӧ�ܵĻ�ѧ����ʽΪ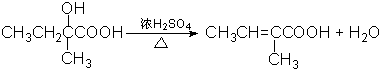
 ��
��
��4��д��E���ڷ����廯�������е�ͬ���칹��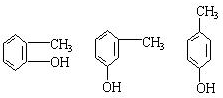
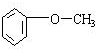

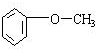 ��
��
����ʽΪC12H14O2��F�л���㷺�����㾫�ĵ������
��֪����R-CH2X+NaOH
| �� |
��

Ϊ�˺ϳɸ��ijʵ���ҵĿƼ���Ա��������кϳ�·�ߣ�

�Իش��������⣺
��1��A�����ں˴Ź����������ܳ���
4
4
�ַ壻C���ʵĹ������������ǻ����Ȼ�
���ǻ����Ȼ�
��E���ʵĽṹ��ʽ

��2�������ϳ�·��������ȡ����Ӧ����
�ڢݢ�
�ڢݢ�
�����ţ�����3����Ӧ�ܵĻ�ѧ����ʽΪ


��4��д��E���ڷ����廯�������е�ͬ���칹��

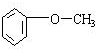

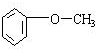
������A����ˮ�������Ǽӳɷ�Ӧ�����ݼӳɲ�����ж�A�Ľṹ��ʽΪCH2=C��CH3��CH2CH3����Ӧ����ˮ�ⷴӦ��������B�Ľṹ��ʽΪCH3CH2C��CH3��OHCH2OH��B�����õ�C����C�Ľṹ��ʽΪCH3CH2C��CH3��OHCOOH������C��D�ķ���ʽ�Ŀ��жϣ���Ӧ������ȥ��Ӧ����D�Ľṹ��ʽΪCH3CH=C��CH3��COOH����Ӧ������±������ˮ�ⷴӦ����E�Ľṹ��ʽΪ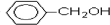 ��E��Dͨ��������Ӧ����F����F�Ľṹ��ʽΪ
��E��Dͨ��������Ӧ����F����F�Ľṹ��ʽΪ ������л���Ľṹ�����ʽ����⣮
������л���Ľṹ�����ʽ����⣮
 ��E��Dͨ��������Ӧ����F����F�Ľṹ��ʽΪ
��E��Dͨ��������Ӧ����F����F�Ľṹ��ʽΪ ������л���Ľṹ�����ʽ����⣮
������л���Ľṹ�����ʽ����⣮����⣺A����ˮ�������Ǽӳɷ�Ӧ�����ݼӳɲ�����ж�A�Ľṹ��ʽΪCH2=C��CH3��CH2CH3����Ӧ����ˮ�ⷴӦ��������B�Ľṹ��ʽΪCH3CH2C��CH3��OHCH2OH��B�����õ�C����C�Ľṹ��ʽΪCH3CH2C��CH3��OHCOOH������C��D�ķ���ʽ�Ŀ��жϣ���Ӧ������ȥ��Ӧ����D�Ľṹ��ʽΪCH3CH=C��CH3��COOH����Ӧ������±������ˮ�ⷴӦ����E�Ľṹ��ʽΪ ��E��Dͨ��������Ӧ����F����F�Ľṹ��ʽΪ
��E��Dͨ��������Ӧ����F����F�Ľṹ��ʽΪ ��
��
��1��A�Ľṹ��ʽΪCH2=C��CH3��CH2CH3������4����ԭ�ӣ�������4�����շ壬C�Ľṹ��ʽΪCH3CH2C��CH3��OHCOOH�����й��������Ȼ��ʹ��ǻ���E�Ľṹ��ʽΪ ��
��
�ʴ�Ϊ��4���ǻ����Ȼ��� ��
��
��2�����ݹ����ŵı仯��֪������ȡ����Ӧ���Ǣڢݢޣ��ʴ�Ϊ���ڢݢޣ�
��3����Ӧ��ΪCH3CH2C��CH3��OHCOOH��Ũ���������·�����ȥ��Ӧ����Ӧ�ķ���ʽΪ ��
��
�ʴ�Ϊ�� ��
��
��4��E�Ľṹ��ʽΪ �����ڷ����廯����˵�����б�����E���ڷ����廯�������е�ͬ���칹��Ϊ
�����ڷ����廯����˵�����б�����E���ڷ����廯�������е�ͬ���칹��Ϊ
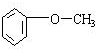 ��
��
�ʴ�Ϊ��
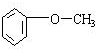 ��
��
 ��E��Dͨ��������Ӧ����F����F�Ľṹ��ʽΪ
��E��Dͨ��������Ӧ����F����F�Ľṹ��ʽΪ ��
����1��A�Ľṹ��ʽΪCH2=C��CH3��CH2CH3������4����ԭ�ӣ�������4�����շ壬C�Ľṹ��ʽΪCH3CH2C��CH3��OHCOOH�����й��������Ȼ��ʹ��ǻ���E�Ľṹ��ʽΪ
 ��
���ʴ�Ϊ��4���ǻ����Ȼ���
 ��
����2�����ݹ����ŵı仯��֪������ȡ����Ӧ���Ǣڢݢޣ��ʴ�Ϊ���ڢݢޣ�
��3����Ӧ��ΪCH3CH2C��CH3��OHCOOH��Ũ���������·�����ȥ��Ӧ����Ӧ�ķ���ʽΪ
 ��
���ʴ�Ϊ��
 ��
����4��E�Ľṹ��ʽΪ
 �����ڷ����廯����˵�����б�����E���ڷ����廯�������е�ͬ���칹��Ϊ
�����ڷ����廯����˵�����б�����E���ڷ����廯�������е�ͬ���칹��Ϊ
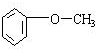 ��
���ʴ�Ϊ��

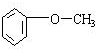 ��
�����������⿼���л�����ƶϣ���Ŀ�Ѷ��еȣ�����ע���A�Ľṹ���ֲ�ȡ���Ƶķ��������ƶϣ����չ����ŵ������Լ������ŵ�ת��Ϊ������Ĺؼ����״���Ϊͬ���칹����жϣ�ע���������Ϣ��

��ϰ��ϵ�д�
�����Ŀ