��Ŀ����
���Ϲ����к���ƻ���ᣬƻ���ᾭ�ۺ����ɾ�ƻ���ᡣ��֪��
a��0.1 molƻ����������NaHCO3��Һ��Ӧ�ܲ���4.48 L CO2����״������
b�� ƻ������ˮ������ʹ��ˮ��ɫ�IJ��
c��RCH2Br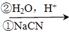 RCH2COOH ��
RCH2COOH ��
d��RC��CH+R��CHO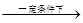 RC��CCH-OH
RC��CCH-OH
R��


��ش�
��1��д��B�Ľṹ��ʽ ��
��2��д��ƻ�������������ŵ����� �� Fת����ƻ������ܷ����ķ�Ӧ���� ��
��3��д����ƻ���������ͬ�����ŵ�ͬ���칹��Ľṹ��ʽ ��
��4��д��F������������Һ��Ӧ�Ļ�ѧ����ʽ ��
��5��д��C��NaOH��Һ��Ӧ�Ļ�ѧ����ʽ ��
��6����ƻ����������õ����������ԣ�����Ϊ��������ߵȲ���Ӧ��������ҽҩ�������������������������ˮ��Ļ�ѧ����ʽ ��
a��0.1 molƻ����������NaHCO3��Һ��Ӧ�ܲ���4.48 L CO2����״������
b�� ƻ������ˮ������ʹ��ˮ��ɫ�IJ��
c��RCH2Br
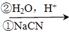 RCH2COOH ��
RCH2COOH ��d��RC��CH+R��CHO
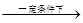 RC��CCH-OH
RC��CCH-OHR��


��ش�
��1��д��B�Ľṹ��ʽ ��
��2��д��ƻ�������������ŵ����� �� Fת����ƻ������ܷ����ķ�Ӧ���� ��
��3��д����ƻ���������ͬ�����ŵ�ͬ���칹��Ľṹ��ʽ ��
��4��д��F������������Һ��Ӧ�Ļ�ѧ����ʽ ��
��5��д��C��NaOH��Һ��Ӧ�Ļ�ѧ����ʽ ��
��6����ƻ����������õ����������ԣ�����Ϊ��������ߵȲ���Ӧ��������ҽҩ�������������������������ˮ��Ļ�ѧ����ʽ ��
��1�� HOOCCH2CH2COOH 1��
��2���ǻ����Ȼ�2�֣���1��
������ȡ����ˮ�� д��1����1�֣����2�֣��д���1�֣�ֱ��0��
��3�� ��1��
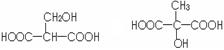
��4��OHCCH2CHClCHO +4 Ag��NH3��2OH NH4OOCCH2CHClCOONH4 +4 Ag��+6NH3+2H2O 2��
NH4OOCCH2CHClCOONH4 +4 Ag��+6NH3+2H2O 2��
��5��HOOCCH2CHClCOOH + 3NaOH NaOOCCH2CH(OH)COONa + NaCl + 2H2O 2��
NaOOCCH2CH(OH)COONa + NaCl + 2H2O 2��
��4��5�����нṹ��ʽд����ȱ�ٲ��ﲻ���֣�δ��ƽ��1�֣�
��6��

��
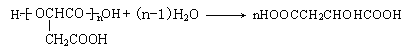
1�֣��ṹ��ʽд����ȱ�ٲ��ﲻ���֣��� ��д�ɡ�
��д�ɡ� ���Ų��۷֣�
���Ų��۷֣�
��2���ǻ����Ȼ�2�֣���1��
������ȡ����ˮ�� д��1����1�֣����2�֣��д���1�֣�ֱ��0��
��3�� ��1��
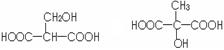
��4��OHCCH2CHClCHO +4 Ag��NH3��2OH
 NH4OOCCH2CHClCOONH4 +4 Ag��+6NH3+2H2O 2��
NH4OOCCH2CHClCOONH4 +4 Ag��+6NH3+2H2O 2����5��HOOCCH2CHClCOOH + 3NaOH
 NaOOCCH2CH(OH)COONa + NaCl + 2H2O 2��
NaOOCCH2CH(OH)COONa + NaCl + 2H2O 2����4��5�����нṹ��ʽд����ȱ�ٲ��ﲻ���֣�δ��ƽ��1�֣�
��6��

��
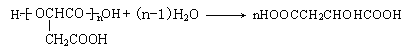
1�֣��ṹ��ʽд����ȱ�ٲ��ﲻ���֣���
 ��д�ɡ�
��д�ɡ� ���Ų��۷֣�
���Ų��۷֣������������1��������������������Ϣ������B�к���-COOH���ṹ��ʽΪHOOCCH2CH2COOH��
��2��ƻ�������������ŵ�����Ϊ�ǻ����Ȼ���Fת����ƻ������ܷ����ķ�Ӧ����Ϊ������ȡ����ˮ�⡣
��3����ƻ���������ͬ�����ŵ�ͬ���칹��Ľṹ��ʽ��
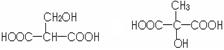 ��
����4��F������������Һ��Ӧ�Ļ�ѧ����ʽΪOHCCH2CHClCHO +4 Ag��NH3��2OH
 NH4OOCCH2CHClCOONH4 +4 Ag��+6NH3+2H2O��
NH4OOCCH2CHClCOONH4 +4 Ag��+6NH3+2H2O����5��C��NaOH��Һ��Ӧ�Ļ�ѧ����ʽΪHOOCCH2CHClCOOH + 3NaOH
 NaOOCCH2CH(OH)COONa + NaCl + 2H2O��
NaOOCCH2CH(OH)COONa + NaCl + 2H2O����6������������ˮ��Ļ�ѧ����ʽΪ
 ��
�����������⿼�����ƻ�����Ӧ�ú��л��ƶϵ����֪ʶ����Ŀ�Ѷȴ����ú�������Ϣ�ǽ���Ĺؼ���

��ϰ��ϵ�д�
�����Ŀ
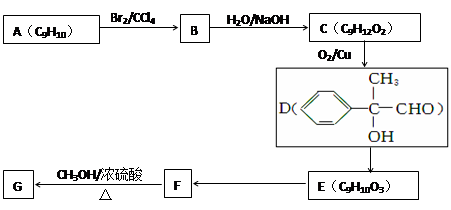
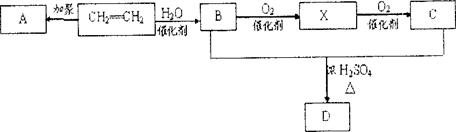
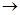 B�ķ�Ӧ����(ѡ��ȡ����Ӧ��ӳɷ�Ӧ) ��
B�ķ�Ӧ����(ѡ��ȡ����Ӧ��ӳɷ�Ӧ) ��
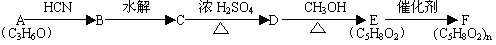
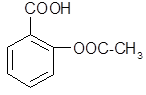
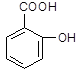 ����B�Ľṹ��ʽΪ ��B�к��еĹ������� ��
����B�Ľṹ��ʽΪ ��B�к��еĹ������� ��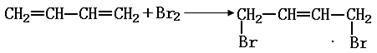
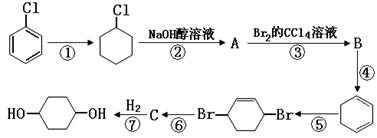

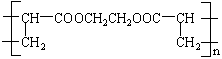 ���ش��������⣺
���ش��������⣺