��Ŀ����
��2013?����һģ���ӷϷ���������Ҫ�ɷ���V2O5��VOSO4��K2SO4��SiO2�ȣ��л���V2O5��һ����������ʾ��ͼ���£���ش��������⣺
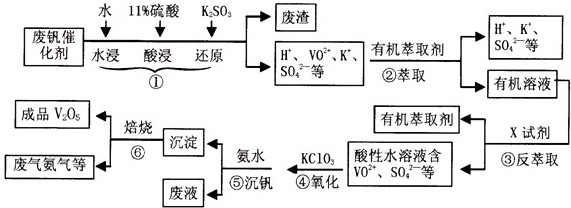
��1��������з�������Ҫ�ɷ���
��2��ʵ�����н�����ȡ��Һ����ʱ��ע����ȡ�������������Һ©������Ȧ�Ͼ��ã���Һ��ֲ�������IJ�����
��3���ڢ۵ı仯���̿ɼ�Ϊ����ʽR��ʾVO 2+��HA��ʾ�л���ȡ����
R2��SO4��n��ˮ�㣩+2nHA���л��㣩?2RAn���л��㣩+nH2SO4��ˮ�㣩
Ϊ��ߢ�����ȡ�ٷ��ʣ�Ӧ��ȡ�Ĵ�ʩ��
��4������ɢ��з�Ӧ�����ӷ���ʽ��
��5��25��Cʱ��ȡ����������������õ��������ʺ���ҺpH֮���ϵ���
����ϱ�����ʵ�������У����м��백ˮ��������Һ�����pHֵΪ
��6���ù��������У�����ѭ�����õ�������

��1��������з�������Ҫ�ɷ���
SiO2
SiO2
������X�Լ�ΪH2SO4
H2SO4
�������ѧʽ����2��ʵ�����н�����ȡ��Һ����ʱ��ע����ȡ�������������Һ©������Ȧ�Ͼ��ã���Һ��ֲ�������IJ�����
��Һ©��ƿ������ƿ�����۶�ƿ��С�ף�������Һ��ӽ������Գ�����������ʱ�رջ������ϲ�Һ��ӷ�Һ©���Ͽڵ�����һ�ձ���
��Һ©��ƿ������ƿ�����۶�ƿ��С�ף�������Һ��ӽ������Գ�����������ʱ�رջ������ϲ�Һ��ӷ�Һ©���Ͽڵ�����һ�ձ���
����3���ڢ۵ı仯���̿ɼ�Ϊ����ʽR��ʾVO 2+��HA��ʾ�л���ȡ����
R2��SO4��n��ˮ�㣩+2nHA���л��㣩?2RAn���л��㣩+nH2SO4��ˮ�㣩
Ϊ��ߢ�����ȡ�ٷ��ʣ�Ӧ��ȡ�Ĵ�ʩ��
������к�����ʹƽ������
������к�����ʹƽ������
����4������ɢ��з�Ӧ�����ӷ���ʽ��
1
1
ClO3-+6
6
VO2++6
6
H-+=6
6
VO3++1Cl-
1Cl-
+3H2O
3H2O
��5��25��Cʱ��ȡ����������������õ��������ʺ���ҺpH֮���ϵ���
| pH | 1.3 | 1.4 | 1.5 | 1.6 | 1.7 | 1.8 | 1.9 | 2.0 | 2.1 |
| ��������% | 88.1 | 94.8 | 96.5 | 98.0 | 98.8 | 98.8 | 96.4 | 93.1 | 89.3 |
1.7-1.8
1.7-1.8
����6���ù��������У�����ѭ�����õ�������
���� �л���ȡ��
���� �л���ȡ��
����������1���������̷�����������Ǽ���ˮ�ܽ⣬�������ܽ�VOSO4������������ػ�ԭV2O5����������л���Һ�м���X�Լ��õ������ӷ�����֪XΪ���
��2�����ݷ�Һ�����IJ�����з����ش�ע���Һ��ԭ���ǡ�������к���ķ��뷽����
��3������ƽ���ƶ�ԭ�������жϣ�������п��������ȡ�ٷ��ʣ�
��4������������ԭ��Ӧ�����غ��ԭ���غ���д��ƽ���ӷ���ʽ��
��5������ͼ�����������백ˮ�ǵ�����ҺPH�������������ʱ��ѣ�
��6���������̣������м���������ڷ�Ӧ���������������ɵ����ʿ���ѭ�����ã�
��2�����ݷ�Һ�����IJ�����з����ش�ע���Һ��ԭ���ǡ�������к���ķ��뷽����
��3������ƽ���ƶ�ԭ�������жϣ�������п��������ȡ�ٷ��ʣ�
��4������������ԭ��Ӧ�����غ��ԭ���غ���д��ƽ���ӷ���ʽ��
��5������ͼ�����������백ˮ�ǵ�����ҺPH�������������ʱ��ѣ�
��6���������̣������м���������ڷ�Ӧ���������������ɵ����ʿ���ѭ�����ã�
����⣺��1��������Ǽ���ˮ�ܽ⣬�������ܽ�VOSO4������������ػ�ԭV2O5����������л���Һ�м���X�Լ��õ���������H+��VO2+��SO42-����֪������Һ�з���������ԭ��Ӧ��XΪ���ᣬ�ʴ�Ϊ��SiO2 ��H2SO4��
��2��ʵ�����н�����ȡ��Һ����ʱ��ע����ȡ�������������Һ©������Ȧ�Ͼ��ã���Һ��ֲ��Һ©��ƿ������ƿ�����۶�ƿ��С�ף�������Һ��ӽ������Գ�����������ʱ�رջ������ϲ�Һ��ӷ�Һ©���Ͽڵ�����һ�ձ��У�
�ʴ�Ϊ����Һ©��ƿ������ƿ�����۶�ƿ��С�ף�������Һ��ӽ������Գ�����������ʱ�رջ������ϲ�Һ��ӷ�Һ©���Ͽڵ�����һ�ձ��У�
��3��R2��SO4��n��ˮ�㣩+2nHA���л��㣩?2RAn���л��㣩+nH2SO4��ˮ�㣩��������к�����Դٽ�ƽ��������У������ȡ�ٷ��ʣ�
�ʴ�Ϊ��������к�����ʹƽ�����ƣ�
��4������Ԫ�ػ��ϼۣ���Ԫ�ػ��ϼ۴�+4�۱仯Ϊ+5�ۣ�˵����Ԫ�ر���������Ԫ�ػ��ϼ۽���������������ԭΪ�����ӣ����������ԭ��Ӧ�����غ��ԭ���غ��ж�ȱ�����ƽ���ӷ���ʽΪ��ClO3-+6VO2++6H+=6VO3++Cl-+3H2O��
�ʴ�Ϊ��1��6��6��6��1 Cl-��3H2O��
��5�������������ʱ��ѣ�����ͼ��������֪��Һ��PH��Ҫ���ڵ�1.7-1.8�����ij�������ʴ�Ϊ��1.7-1.8��
��6�������м���������ڷ�Ӧ���������������ɵ����ʿ���ѭ�����ã���������ͼ��֪���������л���ȡ���ǿ���ѭ�����õ����ʣ�
�ʴ�Ϊ���������л���ȡ����
��2��ʵ�����н�����ȡ��Һ����ʱ��ע����ȡ�������������Һ©������Ȧ�Ͼ��ã���Һ��ֲ��Һ©��ƿ������ƿ�����۶�ƿ��С�ף�������Һ��ӽ������Գ�����������ʱ�رջ������ϲ�Һ��ӷ�Һ©���Ͽڵ�����һ�ձ��У�
�ʴ�Ϊ����Һ©��ƿ������ƿ�����۶�ƿ��С�ף�������Һ��ӽ������Գ�����������ʱ�رջ������ϲ�Һ��ӷ�Һ©���Ͽڵ�����һ�ձ��У�
��3��R2��SO4��n��ˮ�㣩+2nHA���л��㣩?2RAn���л��㣩+nH2SO4��ˮ�㣩��������к�����Դٽ�ƽ��������У������ȡ�ٷ��ʣ�
�ʴ�Ϊ��������к�����ʹƽ�����ƣ�
��4������Ԫ�ػ��ϼۣ���Ԫ�ػ��ϼ۴�+4�۱仯Ϊ+5�ۣ�˵����Ԫ�ر���������Ԫ�ػ��ϼ۽���������������ԭΪ�����ӣ����������ԭ��Ӧ�����غ��ԭ���غ��ж�ȱ�����ƽ���ӷ���ʽΪ��ClO3-+6VO2++6H+=6VO3++Cl-+3H2O��
�ʴ�Ϊ��1��6��6��6��1 Cl-��3H2O��
��5�������������ʱ��ѣ�����ͼ��������֪��Һ��PH��Ҫ���ڵ�1.7-1.8�����ij�������ʴ�Ϊ��1.7-1.8��
��6�������м���������ڷ�Ӧ���������������ɵ����ʿ���ѭ�����ã���������ͼ��֪���������л���ȡ���ǿ���ѭ�����õ����ʣ�
�ʴ�Ϊ���������л���ȡ����
���������⿼���������Ʊ����̵ķ����жϣ����������ʵ�鷽��Ӧ�ã����������е����ʱ仯�ǽ���ؼ�����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 ����ѧҵ���Ե�����ϵ�д�
����ѧҵ���Ե�����ϵ�д�
�����Ŀ
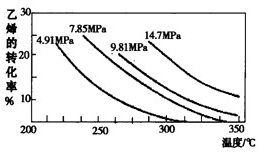 ��2013?����һģ����ҵ�ϲ�����ϩ��ˮ�����ϳ��Ҵ���ԭ��Ϊ��CH2=CH2��g��+H20?CH3CH2OH��g������ͼ����ϩ��ת�������¶ȡ�ѹǿ�ı仯��ϵ������˵����ȷ���ǣ�������
��2013?����һģ����ҵ�ϲ�����ϩ��ˮ�����ϳ��Ҵ���ԭ��Ϊ��CH2=CH2��g��+H20?CH3CH2OH��g������ͼ����ϩ��ת�������¶ȡ�ѹǿ�ı仯��ϵ������˵����ȷ���ǣ�������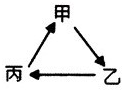 ��2013?����һģ���������и��������У�����֮��ͨ��һ����Ӧ
��2013?����һģ���������и��������У�����֮��ͨ��һ����Ӧ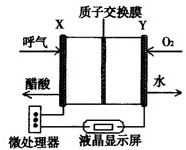 ��2013?����һģ����ͼ������ȼ�ϵ�ؾƾ�����ǣ����缫���Ͼ�ΪPt������˵����ȷ���ǣ�������
��2013?����һģ����ͼ������ȼ�ϵ�ؾƾ�����ǣ����缫���Ͼ�ΪPt������˵����ȷ���ǣ�������