��Ŀ����
����Ŀ����2008����ҹ��Ϸ������ı�ѩ�ֺ��У�ʹ����һ����ѩ��������Ҫ�ɷֵĻ�ѧʽΪXY2��X��Y��Ϊ���ڱ�ǰ20��Ԫ�أ��������Ӻ������ӵĵ��Ӳ�ṹ��ͬ����1mol XY2����54mol���ӡ�
��1������ѩ���Ļ�ѧʽ��____________��X����Ԫ���γɵĻ�����ĵ���ʽ��_______________��
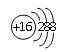
��2��Ԫ��D��Eԭ�ӵ�����������������Ӳ�����2����D��Y���ڣ���D�����ӽṹʾ��ͼ��____________��D��E���γ�һ�ֽṹ����CO2����ԭ�ӷ��ӣ���ÿ��ԭ�Ӿ��ﵽ8e�ȶ��ṹ���÷��ӵĽṹʽΪ________________��
��3��Z��Yͬ���壬Z��Yλ���������ڣ���Z��ԭ�Ӱ뾶����Z���ʳ����³�_____̬���������Һ���̡�����
��4��Ԫ��W��Dͬ���壬��W��D���γ����ֳ��������������ʹƷ����Һ��ɫ����____________���ѧʽ����д������Z���ʵ�ˮ��Һ��Ӧ�Ļ�ѧ����ʽ��____________________��
��5��Ԫ��R��Yͬ���壬���⻯�������ڿ�ʴ������R2��NaOH��Һ��Ӧ�IJ���֮һ��OR2��RΪ1�ۣ����÷�Ӧ�����ӷ���ʽΪ_________________________________��
���𰸡� CaCl2 ![]()
 S=C=S Һ SO2 Br2+SO2+2H2O=2HBr+H2SO4 2F2+2OH-=2F-+OF2+H2O
S=C=S Һ SO2 Br2+SO2+2H2O=2HBr+H2SO4 2F2+2OH-=2F-+OF2+H2O
��������54��3��18������XY2���������Ӻ����������Ϊ18��XΪCa��YΪCl����
��1������ѩ���Ļ�ѧʽ��CaCl2��X����Ԫ���γɵĻ�������CaH2���������Ӽ������ӻ��������ʽ��![]() ����2��Ԫ��D��Eԭ�ӵ�����������������Ӳ�����2����D��Y���ڣ���D��S��E��C�������ӽṹʾ��ͼ��
����2��Ԫ��D��Eԭ�ӵ�����������������Ӳ�����2����D��Y���ڣ���D��S��E��C�������ӽṹʾ��ͼ��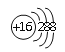 ��D��E���γ�һ�ֽṹ����CO2����ԭ�ӷ��ӣ���ÿ��ԭ�Ӿ��ﵽ8e�ȶ��ṹ���÷�����CS2���ṹʽΪ S=C=S����3��Z��Yͬ���壬Z��Yλ���������ڣ���Z��ԭ�Ӱ뾶�����Z��Br����Z���ʳ����³�Һ̬����4��Ԫ��W��Dͬ���壬��W��D���γ����ֳ��������������ʹƷ����Һ��ɫ����SO2����������������ԣ��ܰ�SO2����Ϊ���ᣬˮ��Һ��Ӧ�Ļ�ѧ����ʽΪBr2+SO2+2H2O=2HBr+H2SO4����5��Ԫ��R��Yͬ���壬���⻯�������ڿ�ʴ������R��F��F2��NaOH��Һ��Ӧ�IJ���֮һ��OF2��RΪ1�ۣ�����˸���ԭ���غ�͵��ӵ�ʧ�غ��֪�÷�Ӧ�����ӷ���ʽΪ2F2+2OH=2F+OF2+H2O��
��D��E���γ�һ�ֽṹ����CO2����ԭ�ӷ��ӣ���ÿ��ԭ�Ӿ��ﵽ8e�ȶ��ṹ���÷�����CS2���ṹʽΪ S=C=S����3��Z��Yͬ���壬Z��Yλ���������ڣ���Z��ԭ�Ӱ뾶�����Z��Br����Z���ʳ����³�Һ̬����4��Ԫ��W��Dͬ���壬��W��D���γ����ֳ��������������ʹƷ����Һ��ɫ����SO2����������������ԣ��ܰ�SO2����Ϊ���ᣬˮ��Һ��Ӧ�Ļ�ѧ����ʽΪBr2+SO2+2H2O=2HBr+H2SO4����5��Ԫ��R��Yͬ���壬���⻯�������ڿ�ʴ������R��F��F2��NaOH��Һ��Ӧ�IJ���֮һ��OF2��RΪ1�ۣ�����˸���ԭ���غ�͵��ӵ�ʧ�غ��֪�÷�Ӧ�����ӷ���ʽΪ2F2+2OH=2F+OF2+H2O��

 �����Ļ������������������ϵ�д�
�����Ļ������������������ϵ�д�����Ŀ����18�֣��̷���FeSO47H2O��������ȱ����ƶѪҩƷ����Ҫ�ɷ֣���ͼ����������м�����������������������ʣ�Ϊԭ�����������̷���һ�ַ�����
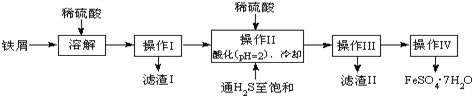
��ѯ���ϣ����й����ʵ��������£�
25��ʱ | pHֵ | 25��ʱ | pHֵ |
����H2S��Һ | 3.9 | FeS��ʼ���� | 3.0 |
SnS������ȫ | 1.6 | FeS������ȫ | 5.5 |
��1��д��Fe��Fe2O3��������Һ�з�����Ӧ�����ӷ���ʽΪ____�� ______ �� __________;
��2������II�У�ͨ�����������͵�Ŀ���ǣ�����ȥ��Һ�е�Sn2+���ӣ���_________��������Һ���������ữ��pH=2��Ŀ����___________��
��3������IV��˳������Ϊ_____________��____________������ϴ�ӣ�
��4������IV�õ����̷�������������ˮϴ�ӣ���Ŀ���ǣ�����ȥ������渽�ŵ���������ʣ���___________��
��5�������£�Ksp[Fe(OH)2]=1.64��10��14������÷�Ӧ��Fe2++2H2O![]() Fe(OH)2+2H+��ƽ�ⳣ��Ϊ��_______________������1λС����;
Fe(OH)2+2H+��ƽ�ⳣ��Ϊ��_______________������1λС����;